Erosions and Ulcers
Libby Edwards
Peter J. Lynch
Although many clinicians do not differentiate erosions from ulcers, this distinction is very useful in the diagnosis of an underlying etiology. The conditions that produce erosions are different from those that produce ulcers.
Erosions and ulcers occur when tissue is lost from the surface of the skin. They are differentiated on the basis of depth. Tissue loss limited to the epithelium produces erosions, whereas tissue loss that extends into, or through, the dermis is termed an ulcer (see Chapter 2 for further discussion of this terminology). The base of an erosion either may be red or may be covered by a loosely adherent yellow crust. The base of an ulcer, on the other hand, may be red or may be covered with a crust that has red, blue, or black heme pigment due to the destruction of blood vessels within the dermis. The crusts covering ulcers may also have considerable fibrin in them and for this reason are adherent and tenacious; this is sometimes termed eschar formation.
There is overlap between erosive and ulcerative diseases, as both early and healing phases of ulcers are erosive, and secondarily infected or manipulated erosions can ulcerate.
Erosions
Erosive and blistering disorders are often morphologically indistinguishable when occurring on genital skin. When blisters occur on fragile skin such as the genital area, the blister is unroofed quickly, producing a shallow erosion. Usually, the blistering nature is appreciated by the round shape of the erosion, or the arcuate configuration of coalescing blisters. Those diseases produced by denuded blisters are discussed in Chapter 10.
Erosions also result from trauma, from epithelial necrosis produced by intense inflammation, or from necrosis resulting from tumor. Those resulting from trauma such as an excoriation are usually linear or angular. Erosive skin diseases can be infectious, immune mediated, or malignant or can arise from excoriations from scratching, as in some vulvar dermatoses.
Erosive Lichen Planus
Lichen planus is a skin disease that exhibits variable morphologies on genital skin. Erosive lichen planus is the most common noninfectious erosive condition occurring on the vulva, although it is less common on the penis and almost nonexistent in circumcised men. Red papules (see Chapter 7) are more common than are erosions in men, and wet skin often exhibits white skin lesions (see Chapter 8). Many patients exhibit more than one form of lichen planus. There are few data on genital lichen planus. Since most patients with genital lichen planus also experience the better studied oral lichen planus, those studies are referenced in this chapter.
Clinical Presentation
Erosive vulvovaginal lichen planus affects adults only, and women are far more often affected by the erosive form, particularly in the United States where circumcision is regularly performed on most newborn boys. The vast majority of women with genital lichen planus have no involvement of extragenital, extramucosal sites. Patients often report genital pruritus, but no pleasure with scratching, and the predominant complaint is that of burning, irritation, rawness, dysuria, dyspareunia, and postcoital bleeding. Many patients with erosive genital lichen planus also report mouth pain, especially with hard food, such as chips, and spicy foods and acidic foods.
Although lichen planus manifests with many different morphologic appearances, classic lesions on keratinized skin are pruritic, violaceous, flat-topped papules (see Chapter 7). However, on mucous membranes, the most common morphologies are erosions or white lacy papules and plaques (see Chapter 8). The white and eroded lesions can present separately or together. In 1982, Pelisse and associates described the vulvovaginal gingival syndrome, introducing erosive lichen planus as a separate subgroup in women. Characteristically, these patients suffer erosions of the vulva, vagina, and oral mucosa, especially the gingivae. Involvement of the modified mucous membranes of the vulva is usual, with bright red,
thin epithelium and erosions most marked in the vestibule. These can be nonspecific, but surrounding white epithelium and reticulate white lacy plaques sometimes reveal the diagnosis of lichen planus (Figs. 11.1, 11.2, 11.3, 11.4 and 11.5). Chronic erosive lichen planus eventuates in scarring (Fig. 11.6). Labial resorption and scarring of the clitoral hood over the clitoris are common. Narrowing of the introitus is also a frequent complication.
thin epithelium and erosions most marked in the vestibule. These can be nonspecific, but surrounding white epithelium and reticulate white lacy plaques sometimes reveal the diagnosis of lichen planus (Figs. 11.1, 11.2, 11.3, 11.4 and 11.5). Chronic erosive lichen planus eventuates in scarring (Fig. 11.6). Labial resorption and scarring of the clitoral hood over the clitoris are common. Narrowing of the introitus is also a frequent complication.
 FIG. 11.1. This photograph illustrates the most common morphology for lichen planus: a nonspecific, well-demarcated erosion of the vestibule with surrounding white epithelium. |
 FIG. 11.2. This erosion also shows a thin rim of hypopigmentation, but the white epithelium extends up skin creases, showing that the hypopigmentation is not simply reactive to an adjacent erosion. |
Vaginal erosions are common with erosive vulvar lichen planus (Fig. 11.7). Even when vulvar lichen planus is papular rather than erosive, accompanying inflammatory vaginitis produced by lichen planus is common (see Chapter 15). Erosive vaginal lichen planus produces purulent vaginal secretions that incite an irritant contact dermatitis in the vestibule. Vaginal adhesions occur with severe disease, sometimes producing obliteration of the
vaginal space that makes sexual intercourse or the introduction of a speculum impossible.
vaginal space that makes sexual intercourse or the introduction of a speculum impossible.
 FIG. 11.5. Uncircumcised men with erosive lichen planus usually exhibit crusting rather than erosions because the skin is drier. |
Penile lichen planus is more commonly associated with lichen planus at other cutaneous sites than is vulvovaginal lichen planus. Lesions are less often eroded, but when erosions occur, they are very painful (Figs. 11.8 and 11.9). These erosions may be associated with oral lesions and run a similar prolonged clinical course to that seen in women (1). Men with erosive penile lichen planus also experience scarring, with phimosis if uncircumcised, or obliteration of the sharp distinction of the glans from the shaft in the circumcised penis. Perianal lesions sometimes occur in both genders (Fig. 11.10).
 FIG. 11.6. Scarring on the basis of lichen planus can be remarkable, sometimes producing complete closure of the vagina and the introitus, which is threatening this patient. |
 FIG. 11.7. Unlike lichen sclerosus, lichen planus usually affects the vagina, manifested by generalized mucosal erythema or erosions, as well as an inflammatory wet mount with parabasal cells. |
Gingival lesions may be localized or generalized, primarily manifested by tender erosions and desquamative gingivitis, sometimes with surrounding solid or reticulate white papules (Fig. 11.11). Often, linear reticulate papules or erosions occur on the buccal mucosa, and the tongue is often affected as well (Figs. 11.12 and 11.13). Esophageal lichen planus is increasingly recognized and is more common than the literature suggests. Strictures producing dysphagia and weight loss are suggestive of this involvement, and squamous cell carcinoma of the esophagus is a serious complication (2). Conjunctival involvement and lacrimal duct scarring can occur (1).
Squamous cell carcinoma sometimes occurs in oral, vulvar, and penile lichen planus. Indurated erosions and ulcers should be biopsied, as should chronic hyperkeratotic areas.
 FIG. 11.8. This typical erosion of lichen planus on the glans and inner foreskin is at risk for producing phimosis. |
There have been reports for many years of lichen planus occurring more often in patients with hepatitis C as well as reports discounting this association. A recent survey of the literature suggests a statistically significant association with hepatitis C in patient with oral lichen planus (3,4). More recently, a study has noted an association of oral lichen planus and hypothyroidism (5).
Diagnosis
The diagnosis of lichen planus can be made with confidence when erosions are accompanied by white, lacy, reticulate, or fernlike papules of the genitalia or mouth. Otherwise, a biopsy is required, although most often the histology is returned as “lichenoid dermatitis” consistent with but not diagnostic of lichen planus. Lichen planus and a lichenoid medication reaction can produce identical clinical and histologic picture. The biopsy should be taken from the edge rather than the center of an erosion, and it should be submitted for routine histology. If the biopsy is very nonspecific or is suggestive of an autoimmune blistering disease such as benign mucous membrane pemphigoid or pemphigus vulgaris, an additional biopsy submitted for direct immunofluorescence is necessary. This biopsy should be obtained from normal, perilesional skin and stored in transport media rather than formalin.
 FIG. 11.12. These white, interlacing striae on the buccal mucosa are pathognomonic for mucosal lichen planus and the most common manifestation of oral lichen planus. |
 FIG. 11.13. Erosive genital lichen planus is sometimes associated with lichen planus of the dorsal and lateral tongue. This can be smooth, with loss of papillae, erosive, or hyperkeratotic. |
Histologic features of erosive lichen planus include a dense, lymphocytic, dermal bandlike infiltrate extending to and damaging the basal layer (Fig. 11.14). A prominent granular cell layer, hyperkeratosis, and acanthosis are common, but erosive mucous membrane lichen planus often exhibits epithelial thinning and flattening. A biopsy of eroded skin that does not include epithelium cannot demonstrate a diagnosis of lichen planus, so biopsy sampling should include the epithelialized edge of an erosion. Colloid and Civatte bodies are frequently seen in the lower epidermis and upper dermis, and a direct immunofluorescent biopsy makes these more easily visualized.
 FIG. 11.14. A biopsy of mucosal lichen planus usually shows a lichenoid infiltrate of chronic inflammatory cells in the upper dermis abutting and destroying the basal cell layer. |
Most chronic erosive skin diseases are in the differential diagnosis of erosive genital lichen planus. Pemphigus vulgaris and benign mucous membrane pemphigoid can be nearly indistinguishable, but these are far, far less common and usually more easily diagnosed by routine and direct immunofluorescent biopsy. Often, surrounding white epithelium suggests the diagnosis of erosive lichen planus, but any chronic erosion can induce surrounding nonspecific white epithelium. Sometimes, this white epithelium can resemble lichen sclerosus, but lichen sclerosus rarely affects the vagina, and it shows a characteristic crinkled or occasionally waxy texture unlike that of lichen planus. Toxic epidermal necrolysis, severe erosive candidiasis, and an irritant contact dermatitis to an agent such as podophyllum resin can mimic erosive lichen planus, but these are acute rather than chronic in onset. Like erosive lichen planus, plasma cell mucositis also exhibits red, moist, glistening papules, and plaques that may appear erosive. The histology differentiates lichen planus from plasma cell vulvitis or balanitis and from the erosive genital lesions of graft versus host disease that may also be associated with lichenoid skin lesions.
EROSIVE LICHEN PLANUS
Diagnosis
Nonspecific chronic erosions of mucous membranes or modified mucous membranes statistically indicate the diagnosis.
Pathognomonic lacy, white surrounding striae confirm diagnosis.
White lacy striae of the mouth strongly suggest the diagnosis.
In the absence of white lacy striae or a possible offending drug, a biopsy of the edge of an erosion reporting lichenoid mucositis or dermatitis gives presumptive diagnosis.
Pathophysiology
Lichen planus is an immunologic disease associated with T-cell subset disturbance, although specifics are poorly known, and a recent study noted the presence of mast cells (6). Multiple studies have examined various aspects of more specific abnormalities of immune factors. This may be a heterogeneous condition, and patients with class II HLA DQB1*0201 allele are more likely to develop more widespread disease, including esophageal, lacrimal duct, and extra-auditory canal involvement (7). The management of lichen planus consists primarily of the use of immunosuppressant medications.
Management
The treatment of erosive lichen planus often is challenging. Although most disease improves substantially
with local care and superpotent corticosteroids, more severe disease requires the systemic use of systemic antimetabolites and immunosuppressive agents with variable success. Circumcision is sometimes curative in men (6). Data regarding the benefit of therapy are poor. A recent Cochrane Database review showed convincing evidence for benefit of any therapies (8); however, clinicians see very significant improvement in patients with some therapies, especially corticosteroids. Although incurable, lichen planus in most patients is managed quite well with topical immunosuppressive therapy.
with local care and superpotent corticosteroids, more severe disease requires the systemic use of systemic antimetabolites and immunosuppressive agents with variable success. Circumcision is sometimes curative in men (6). Data regarding the benefit of therapy are poor. A recent Cochrane Database review showed convincing evidence for benefit of any therapies (8); however, clinicians see very significant improvement in patients with some therapies, especially corticosteroids. Although incurable, lichen planus in most patients is managed quite well with topical immunosuppressive therapy.
Nonspecific therapy
Avoidance of irritants such as soap and overwashing, panty liners, and unnecessary topical medications is useful. Topical emollients such as petrolatum are soothing, as are measures such as air-drying rather than towel-drying. Because vulvovaginal lichen planus occurs primarily in the postmenopausal age group, topical or systemic estrogen replacement can be crucial, avoiding an additional and easily corrected cause for mucosal thinning.
Topical therapy
First-line specific therapy for lichen planus consists of corticosteroid therapy. Superpotent topical steroid ointments (e.g., clobetasol propionate 0.05%, halobetasol propionate 0.05%) applied to vulvar or penile lesions twice daily can be beneficial. Lichen planus of the uncircumcised glans that does not respond adequately can be treated with corticosteroids under condom occlusion for short periods with careful follow-up. Creams, gels, lotions, and solutions are to be avoided because of the irritation induced by the alcohols regularly contained in these vehicles.
This medication can also be inserted intravaginally at night, as can 25 mg hydrocortisone acetate rectal suppositories or hydrocortisone foam. Recalcitrant vaginal disease can be treated with 100 to 200 mg compounded hydrocortisone suppositories or with insertion of potent corticosteroids into the vagina. However, significant absorption can occur, so careful follow-up is again required. Also, intravaginal corticosteroids sometimes precipitate vaginal candidiasis, so weekly fluconazole, twice- or thrice-weekly insertion of a topical antifungal cream, or a warning to the patient to call if sudden itching occurs is important.
The mouth should be addressed as well, and this author often finds oral lichen planus to be a greater challenge than genital lichen planus. There are several options for the delivery of a corticosteroid to the mouth. Nystatin oral suspension and dexamethasone elixir can be mixed half and half, and then used in a swish, hold as long as possible, and spit fashion. The nystatin helps to prevent candidiasis in the face of corticosteroid use. Alternatively, clobetasol gel 0.05% can be applied to affected areas of the mouth. Both are begun with four times a day dosing and then decreased to the least frequent dosing required to control disease.
Second-line therapy consists of the topical calcineurin inhibitors pimecrolimus 1% cream and, especially, tacrolimus 0.1% ointment with the caveat that these medications are not approved by the U.S. Food and Drug Administration (FDA) for lichen planus, are expensive, and often produce unacceptable and long-lasting burning when applied to inflamed genital skin (9,10). In addition, improvement is delayed when compared to topical corticosteroids, and they are black-boxed by the FDA for a risk of producing skin cancer and lymphoma, worrisome for patients who are already at risk for transformation to squamous cell carcinoma. However, most genital dermatologists feel that uncontrolled erosive mucosal lichen planus has a substantially higher risk for squamous cell carcinoma than does the use of a calcineurin inhibitor.
Discomfort with application and cost can be minimized by compounding tacrolimus, diluting a 1 mg tacrolimus capsule in a liter of water, and applying this twice a day and using this as a swish, hold, and spit medication four times a day initially for oral lichen planus. Tacrolimus suppositories for the vagina can be compounded as well, and compounding pharmacies have these recipes.
EROSIVE LICHEN PLANUS
Management
Topical therapy
Superpotent corticosteroid ointment twice daily
Calcineurin inhibitors (tacrolimus or pimecrolimus) twice daily
For postmenopausal women with vaginal lichen planus—estrogen replacement
Intralesional corticosteroids
Triamcinolone acetonide 10 mg/mL into recalcitrant lesions
Systemic therapy occasionally
Hydroxychloroquine 200 mg orally twice daily
Methotrexate up to 25 mg orally weekly
Mycophenolate mofetil up to 1,500 mg twice daily
Tumor necrosis factor-alpha blockers
Local supportive care—dilators, infection control, avoidance of irritants
Intralesional therapy
Corticosteroids injected into recalcitrant lesions sometimes produce very nice improvement (see Chapter 4). Triamcinolone acetonide 10 mg/mL is the usual preparation, and the amount depends upon the area to be injected. The oral epithelium can be anesthetized with an oral anesthetic compounded by dentists to produce
prompt anesthesia, resulting in a nearly painless injection. However, this topical anesthetic should be avoided on genital skin, where it sometimes produces sloughing.
prompt anesthesia, resulting in a nearly painless injection. However, this topical anesthetic should be avoided on genital skin, where it sometimes produces sloughing.
Systemic therapy
At times, topical and intralesional therapies do not produce adequate improvement. These patients benefit from the addition of systemic immunosuppressive therapy to topical medications.
Systemic corticosteroids (prednisone 40 to 60 mg/day) effect significant and rapid improvement in most patients, but the condition recurs when the medication is stopped. This can be used initially in patients with severe erosions to clear skin sufficiently before transitioning to topical therapy. Alternatively, corticosteroids can be administered by intramuscular injection with triamcinolone acetonide, 40 to 80 mg. One regimen consists of a monthly injection for 3 months in an attempt to interrupt the disease process and trigger long-lasting benefit ([personal communication, Lynette Margesson, MD], and the severity of long-term side effects prevents this from being a useful treatment). In general, prompt relapse occurs after discontinuation of systemic corticosteroid therapy
The onset of benefit of all other systemic treatment is slow, requiring about 3 months to produce full effect, and other therapies improve lichen planus less predictably. Each should be used at full dose for 3 months before. There are no controlled trials for these medications, but only anecdotal reports. Hydroxychloroquine 200 mg twice a day is the least expensive therapy, with minimal laboratory monitoring. Methotrexate 25 mg weekly is another cost-effective therapy, but supported by only anecdotal reports (11). There are allusions to benefit from oral retinoids such as isotretinoin 40 to 80 mg/day and acitretin at about 25 mg/day, but side effects including teratogenicity require careful monitoring. I have not found these to be useful. Other systemic treatments tried include oral griseofulvin at 500 mg twice daily, azathioprine 75 mg twice a day, cyclophosphamide, and mycophenolate mofetil, with anecdotal benefit in some patients; clinician familiarity with these medications for appropriate monitoring is important. Thalidomide at 100 to 150 mg/day is a theoretical potential therapy (12). More recently, tumor necrosis factor (TNF) antagonists have been used with some favorable case reports, but even more reports of lichenoid and psoriasiform eruptions produced by this class of medication (13). No one therapy is ideal, and few produce consistent improvement without significant risks or side effects. I have found methotrexate and adalimumab to offer partial but welcome relief in some patients with severe disease uncontrolled by topical medications. I have not observed improvement with other systemic medications.
Surgery
Circumcision is the only surgical procedure that sometimes improves the mucosal lichen planus. However, surgery is occasionally needed to separate vaginal adhesions, to reverse tightening of the introitus, or to uncover a buried clitoris. Narrowing of the introitus occasionally progresses to complete closure, resulting in urinary retention requiring urgent surgery. Skin disease should be controlled as well as possible before surgery is contemplated, and great care has to be taken postoperatively to prevent rapid scar reformation. Use of topical steroids and dilators is advisable.
Erosive anogenital lichen planus is chronic and painful, and there are no standard treatment measures that help all patients. Nor are there any controlled trials that evaluate therapy for anogenital lichen planus. However, experience suggests that there are various therapies that help some patients. Occasionally, erosive anogenital lichen planus remits, but this is the exception. Also, because of the risk of squamous cell carcinoma, patients should be checked regularly throughout the course of the disease. Vulvologists have noted residual pain in women successfully treated for erosive vulvovaginal lichen planus, showing that lichen planus can trigger vulvodynia.
Plasma Cell Balanitis and Vulvitis
The origin of Zoon balanitis or vulvitis (also called balanitis or vulvitis circumscripta plasmacellularis, inflammatory erythroplasia) is unknown, although some physicians believe that plasma cell vulvitis and balanitis are related to lichen planus. This condition is discussed primarily in Chapter 6.
Plasma cell vulvitis and balanitis present as a glistening, red, usually solitary plaque on the glans penis or vulva. Although this often appears erosive, the lesion generally is atrophic and red but intact (Figs. 11.15 and 11.16). This may be asymptomatic or may present with burning, irritation, or itching. The diagnosis is made by the appearance and confirmed on biopsy.
In men, circumcision is curative. Otherwise, potent topical corticosteroids offer some symptomatic relief and improvement in appearance in many patients, but relapse is the rule on discontinuation of treatment. Topical tacrolimus, pimecrolimus, and masoprocol have been useful in some, but reported results of therapy are conflicting (14,15,16). Topical corticosteroids mixed with fusidic acid, imiquimod, intralesional corticosteroids, and CO2 laser have been reported beneficial at times (17,18,19,20).
There is one report of squamous cell carcinoma in situ (intraepithelial neoplasia) in a patient with plasma cell balanitis, so that ongoing surveillance is recommended (21).
Genital Skin Fold Fissures
Genital skin fold fissures are not a disease, but a common clinical presentation of several genital diseases. Genital fissures, especially fissures of the vulva, produce stinging
pain that patients often refer to as feeling like paper cuts. These erosions are often recurrent or chronic.
pain that patients often refer to as feeling like paper cuts. These erosions are often recurrent or chronic.
 FIG. 11.15. Plasma cell vulvitis, also called Zoon vulvitis, presents as well-demarcated brown-red or bright-red, purpuric patches of the vestibule or medial labia minora. |
Clinical Presentation
Skin fold fissures consist of linear erosions within skin folds, especially the interlabial folds, edges of the clitoral hood in women, in the coronal sulcus and skin wrinkling of the penile shaft, and within the normal skin markings of the perineal body and perianal skin of both men and women (Figs. 11.17, 11.18, 11.19 and 11.20). These are so transient that the diagnosis is often made by the history in association with thin, red lines in the areas of fissuring or by having the patient return as soon as fissures recur. Sometimes, magnification is required for identification. At other times, the fissures are so deep as to actually represent ulcers.
Both skin fold fissures and posterior fourchette fissuring present with a tearing, stinging, burning sensation. The stinging often is accentuated when urine, semen, medicated creams, or even water touches the area. The patient often reports prompt healing, but recurrence with sexual activity, rubbing, or scratching.
 FIG. 11.18. At times, these fissures can be very subtle but painful and heal quickly, so that careful examination with good lighting is often required for visualization. |
Diagnosis
The diagnosis of skin fold fissuring is made by its morphology. The diagnosis of the underlying cause is more difficult and requires the elimination or treatment of each individual cause. The diagnosis of the underlying cause provides the diagnostic dilemma in this condition.
Pathophysiology
Skin fold fissures are nonspecific linear erosions with any irritation, often exacerbated by or precipitated by sexual activity. The most common underlying irritant to cause these is Candida albicans infection. When yeast is the primary cause, the gluteal cleft is often prominently involved.
Tinea cruris does not often produce fissures, because this is a disease of dry, keratinized skin rather than the vulva, penis, or perianal skin. Other infections that can cause fissuring are recurrent herpes simplex virus (HSV) infection and vulvar or vaginal bacterial infection, most often Staphylococcus aureus or Streptococcus, either α-hemolytic or group B Streptococcus. Although group B Streptococcus is nearly always an asymptomatic normal colonizer, occasionally it can contribute to vulvar irritation and fissuring.
 FIG. 11.20. Fissures (arrow) are much less common in men. This patient was irritated by his partner’s personal lubricant. |
Any itchy dermatosis, such as eczema (lichen simplex chronicus), lichen sclerosus, or psoriasis, can produce these skin fold fissures, which, in turn, increase itching, beginning or maintaining an itch-scratch cycle. Fissuring of the penis has been noted in association with penile intraepithelial neoplasia (Bowen disease).
Management
For skin fold fissures, the identification, treatment, and suppression of the underlying condition are crucial. When no underlying factors are identified, a trial of an antistaphylococcal or streptococcal antibiotic such as cephalexin, a topical corticosteroid ointment such as triamcinolone acetonide 0.1%, and an antifungal therapy such as fluconazole 150 mg every 4 to 7 days (or nystatin ointment, because creams may burn eroded skin) often produces significant control of fissures. After the skin has healed, the medications can be gradually discontinued, although recurrence is common.
Posterior Fourchette Vulvar Fissures Clinical Presentation
Posterior fourchette fissures occur most often in premenopausal women and almost exclusively in sexually active individuals. The onset is usually sudden, without preceding trauma or infection; burning and splitting of the posterior fourchette occur during sexual intercourse. Semen, water, and urine sting. The area usually heals quickly, but fissure recurs with most acts of intercourse.
On clinical examination, there is a linear erosion at the midline posterior fourchette that may be thin and subtle or relatively wide, obvious, and nearly ulcerative (Figs. 11.21 and 11.22). Some patients exhibit fairly long-lasting erythema of the area after healing. Posterior fourchette fissuring is easily seen when the patient presents within a day or two following sexual intercourse. Rarely, women experience erosions or ulcerations that are not at the 6 o’clock position (Fig. 11.23).
Some women report pain around the fissure but within the vestibule. Some clinicians postulate that fissuring occurs in association with vulvodynia, or vestibulodynia (see Chapter 5).
Diagnosis
The diagnosis of a recurrent posterior fissure is easily made by the morphology and history. Diseases to consider in the differential include vestibulodynia (vulvar
vestibulitis syndrome) because of the history of posterior vestibular pain with intercourse; examination of the patient the day following sexual activity reveals the presence of the fissure. Also to be considered is HSV infection, because HSV can present as recurrent fissures. The location and immediate occurrence with sexual activity usually suggest the correct diagnosis. Polymerase chain reaction (PCR) for HSV can be performed if necessary.
vestibulitis syndrome) because of the history of posterior vestibular pain with intercourse; examination of the patient the day following sexual activity reveals the presence of the fissure. Also to be considered is HSV infection, because HSV can present as recurrent fissures. The location and immediate occurrence with sexual activity usually suggest the correct diagnosis. Polymerase chain reaction (PCR) for HSV can be performed if necessary.
 FIG. 11.21. Posterior fourchette fissures present with predictable painful splitting during sexual activity. |
MECHANICAL VULVAR FISSURES
Diagnosis
Both history and visualization of a same-site fissure immediately with sexual activity
Pathophysiology
Although some clinicians have implicated low estrogen levels as a cause of posterior fourchette fissuring, the addition of oral or topical estrogen replacement is not helpful. One report describes skin disease often detected on biopsy (22), but a biopsy of the posterior fourchette sometimes eventuates in poor healing, so sampling that avoids the midline is desirable. Some patients have tightness of the skin at the posterior fourchette, predisposing them to this condition. However, an additional factor is probably needed to allow splitting suddenly with intercourse, since many women exhibit this tight skin. Some believe that pelvic floor abnormalities may contribute to both vestibulodynia and posterior fourchette fissuring, helping to explain a possible association of these two conditions. Lichen sclerosus predisposes to posterior fourchette fissuring, and this fissuring resolves with treatment of the lichen sclerosus. Most women simply and relatively suddenly experience the onset of posterior fourchette fissuring with no known cause or accompanying abnormalities.
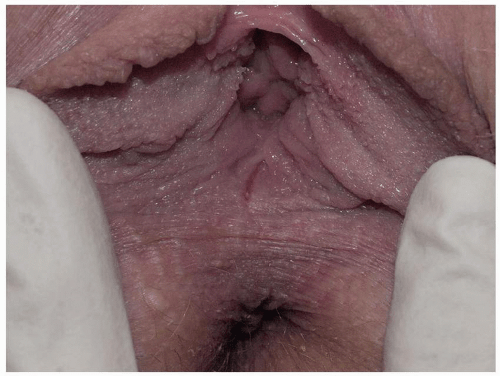 FIG. 11.22. Even very fine mechanical fissures are painful, particularly when semen or urine touches the area. |
Management
The treatment of posterior fourchette fissuring can be difficult. Liberal lubrication during sexual activity, topical anesthetics such as lidocaine jelly 2%, and woman-on-top positioning are adequate therapy for some women.
One management option is to have the patient produce the fissure and then keep it open with frequent insertion of a dilator so that re-epithelialization occurs across the surface of the erosion.
Otherwise, simple lengthwise excision with suture closure of the fissuring area does not effect a cure and in fact often worsens the splitting as the caliber of the introitus is narrowed. However, a perineoplasty is usually curative. The skin surrounding the posterior fourchette is surgically excised, and vaginal epithelium is advanced to cover the defect. The success of the procedure depends largely on patient selection. Women should be evaluated for the
likely presence of vestibulodynia before surgery, since their pain does not totally remit unless the entire vestibule is removed or unless they receive medical treatment and/or physical therapy for vestibulodynia in addition to surgery.
likely presence of vestibulodynia before surgery, since their pain does not totally remit unless the entire vestibule is removed or unless they receive medical treatment and/or physical therapy for vestibulodynia in addition to surgery.
MECHANICAL VULVAR FISSURE
Management
Careful visual evaluation for underlying skin disease such as lichen sclerosus
Replace estrogen if atrophic wet mount
Lubrication before sexual activity
Patient produces the fissure, then keeps it open with careful perineal massage with petroleum jelly and dilators, to allow the fissure to re-epithelialize and heal in the open position
If unsuccessful, perineoplasty; avoid simple excision with suture closure
Erosive Lichen Sclerosus (Lichen Sclerosus et Atrophicus, Hypoplastic Dystrophy)
Lichen sclerosus is a chronic fragile dermatosis characterized by atrophic white plaques, occurring most commonly on the genital skin of both sexes, although there is a female preponderance of 10:1 (see Chapter 8 for primary discussion). Although lichen sclerosus is not primarily an erosive disease, intense itching with resulting rubbing of this fragile skin often produces erosions and purpura. There is fissuring of the anterior midline, as well as vestibular erosions following sexual activity.
The usual presenting symptom of lichen sclerosus is severe pruritus, often leading to painful erosions as this fragile skin is scratched (Fig. 11.24). These excoriations and other erosions resulting from fragility of the skin can be very painful and can predispose to secondary infection. The atrophic skin fissures more easily, so patients may present with pain on intercourse, and young girls often experience constipation resulting from pain of fissures with defecation. Lichen sclerosus is characterized by pallor and thin, crinkled skin; advanced disease is often associated with resorption of the labia minora, a clitoris covered by scar, and narrowing of the introitus. Purpura and hyperkeratosis are common, and lichen sclerosus may spread to perianal skin in women.
Eroded lichen sclerosus is sometimes indistinguishable from erosive lichen planus, and these two diseases are known to coexist in some patients. Erosive and scarring lichen sclerosus can also mimic other chronic erosive or blistering diseases such as benign mucous membrane pemphigoid and pemphigus vulgaris, so biopsy and examination of other mucous membranes and skin surfaces may be necessary to make the diagnosis of lichen sclerosus.
For men, circumcision is often curative. Otherwise, the main treatment is potent topical corticosteroid ointment such as clobetasol propionate 0.05%, applied twice daily until improvement occurs, and then the frequency is reduced. Eroded lichen sclerosus is more likely than is noneroded disease to present with bacterial or candidal superinfection, which should be addressed. In addition, these patients are more likely to develop superinfection after the initiation of an ultrapotent corticosteroid. Early detection and treatment, or even prophylaxis during the first week or so of therapy until the skin improves, can be extremely helpful.
 FIG. 11.24. This woman experienced erosive lichen sclerosus strictly limited to the clitoris. A biopsy of the edge of the erosion, including some white epithelium yielded the correct diagnosis. |
This is a chronic skin disease that requires chronic corticosteroid therapy for ongoing control. Patients should undergo ongoing surveillance to assess the activity of the disease, side effects of medication, and any early signs of malignant transformation.
Lichen Simplex Chronicus (Eczema, Atopic Dermatitis, Neurodermatitis)
This excruciatingly pruritic condition exhibits erosions only secondarily, as a result of excoriation (see also Chapter 6). A genetic tendency to itch in the presence of local irritation initiates an itch-scratch cycle characterized by skin changes produced by rubbing (lichenification, scale, and erythema) and scratching (linear erosions of excoriation) (Figs. 11.25 and 11.26). The diagnosis is generally recognized by the history of scratching and the observation of irregular or linear erosions consistent with excoriations on the vulva or scrotum. Therapy includes topical corticosteroids, infection control, and nighttime sedation to minimize scratching during sleep.
Contact Dermatitis
Contact dermatitis, especially irritant contact dermatitis, sometimes presents with erosion. A strong irritant produces a chemical burn that can either blister or erode. The erosions are in the distribution of contact with the irritant, usually with surrounding erythema (Fig. 11.27). Because the irritant is strong, the identity of the original agent and burning with contact most often is remembered.
Fixed Drug Eruption
A fixed drug eruption, discussed primarily in Chapter 10, is a peculiar allergic reaction to a medication that produces same-site blisters that erode into well-demarcated, often round lesions (Fig. 11.28).
Necrolytic Migratory Erythema
This is a very rare condition that is caused by a glucagonoma, an α-cell tumor of the pancreas, which is usually malignant. Serum levels of glucagon are elevated. Patients
are usually ill with weight loss, diarrhea, malabsorption, and diabetes.
are usually ill with weight loss, diarrhea, malabsorption, and diabetes.
 FIG. 11.28. This well-demarcated, nearly round erosion following a very short-lived blister resulted from a fixed drug eruption to doxycycline. |
The rash begins as erythematous papules around orifices and flexures, including those of the genitalia. These papules coalesce into plaques, and central erosions occur and crust, eventually producing a circinate migratory erythema.
Histology of the skin shows superficial necrolysis and infiltration with lymphocytes. Treatment consists of removal of the tumor, if possible. Topical steroids may alleviate symptoms of the rash, but they do not clear it.
Malignancies Presenting as Erosions
Basal Cell Carcinoma
Basal cell cancers are associated, classically, with sites of sun exposure, but 10% occur on nonexposed sites and account for 5% of genital cancers (see Chapter 5). They occur more frequently in fair than dark-skinned men and women. Basal cell carcinomas are more common with increasing age and often present with itching but only minor pain or soreness. The lesions often display the typical pearly, rolled edge, with a central depression or necrotic erosion (Fig. 11.29), whence the term “rodent ulcer.” They invade locally, but almost never metastasize. Still, local invasion and necrosis of untreated tumors cause significant tissue damage.
Diagnosis and treatment are affected by conservative local excision. Those in more difficult sites may be amenable to radiotherapy.
Invasive Squamous Cell Carcinoma
Squamous cell cancers account for more than 90% of genital cancers and often arise in the site of chronic scarring or inflammation such as lichen sclerosus and lichen planus (see Chapter 5 for primary discussion) or some human papillomavirus infection (Fig. 11.30). Squamous cell carcinomas occur most commonly in the older age group (the mean presenting age is older than 65 years), but it may present at any age from the 20s. The lesions present as red or skincolored plaques or nodules that can erode or ulcerate as they enlarge. There may be associated inguinal lymphadenopathy. Diagnosis is made by biopsy, and treatment is surgical.
Intraepithelial Carcinoma (Differentiated Vulvar/Penile/Scrotal/Anal Intraepithelial Neoplasia, Highgrade Squamous Intraepithelial Lesion, Bowen Disease, Bowenoid Papulosis, Squamous Cell Carcinoma In Situ, Erythroplasia of Queyrat)
Histologic squamous cell carcinoma in situ is termed differentiated intraepithelial neoplasia when associated with lichen sclerosus or lichen planus, or high-grade squamous intraepithelial lesion when associated with human papillomavirus infection (Fig. 11.30) (see Chapter 5 for primary discussion). These conditions can present with multiple morphologies, including skin-colored, white, or brown papules and plaques. The appearances illustrated here are erosive and ulcerative.
Extramammary Paget Disease (see chapter 6 for primary discussion)
Extramammary Paget disease is a rare neoplasm arising from dysplastic secretory glandular adenocarcinoma cells occurring in the anogenital or axillary epidermis or
from underlying carcinoma from urogenital origin (see Chapter 6). Anogenital Paget disease most often is unassociated with malignancy, but there is an associated gastrointestinal or genitourinary malignancy in about 15% of patients.
from underlying carcinoma from urogenital origin (see Chapter 6). Anogenital Paget disease most often is unassociated with malignancy, but there is an associated gastrointestinal or genitourinary malignancy in about 15% of patients.
 FIG. 11.30. Eroded HPV-associated high-grade squamous intraepithelial lesions (previously termed AIN III) has eventuated into ulcerated squamous cell carcinoma inferiorly. |
 FIG. 11.31. Extramammary Paget disease is characterized by a red plaque with superficial erosions and white, hyperkeratotic islands of epithelium. |
Extramammary Paget disease generally presents as well-demarcated, slow-growing, eroded, itchy, red plaques (Fig. 11.31). When pruritus is a prominent feature, the area may become lichenified and may mimic benign dermatitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





















