Fig. 61.1
Representative fluorescent images of naïve Lewis rat epineural sheath. Blue staining (DAPI) represents nuclei, green (FITC) signal represents positive response for stained markers. All photographs were taken under 20× magnification
In peripheral nerves of the monofascicular type, the outer epifascicular epineurium consists of the local vascular network within areolar connective tissue components, disposed among randomly and partly longitudinally arranged bundles of collagen fibrils [10]. The outer layer of the epineural sheath has features of areolar tissue with a well-developed vascular network and it is probable that this outer layer of the epineurium is the source of potential VEGF expression [10].
Anatomical studies of the spinal cord show transmedian fibers decussating posteriorly at all levels of the spinal cord to synapse in the laminae on the contralateral dorsal horn [16, 17]. These anatomical studies provide evidence that an opportunity exists for crosstalk between the left and the right sides of the spinal column. In our prior experiments we demonstrated further evidence of this crosstalk; strong up-regulation of pro-inflammatory cytokines was observed in the contralateral undisturbed DRG 48 h after topical application of chromic gut suture onto an exposed DRG [18]. Dorsal root ganglia covered by the epineural sheath expressed VEGF mRNA at 24 h (Fig. 61.2). However; the contralateral undisturbed DRG in the experimental group (DRG covered by epineurium), expressed half the VEGF mRNA when compared to the contralateral undisturbed DRG in the laminectomy-only group. Based on our observations, it would appear that placement of the epineural sheath onto the DRG attenuates this inflammatory response on the contralateral side. In addition, the DRG covered by an epineural sheath returned to baseline VEGF mRNA expression quicker than the uncovered laminectomy-only DRG, potentially signifying a neuro-protective effect. We hypothesize that this is because the epineural sheath serves as a mechanical biologic barrier. In this role the epineural sheath has the potential to mitigate the effects of inflammation on the DRG. This was demonstrated by negligible expression of CD3 and CD45 72 h after application of the epineaural sheath onto the DRG. In addition to serving as a mechanical barrier, the epineural sheath upregulates VEGF, expresses laminin and vWF and thus has the potential to create a favorable local micro-environment for neuroregeneration.
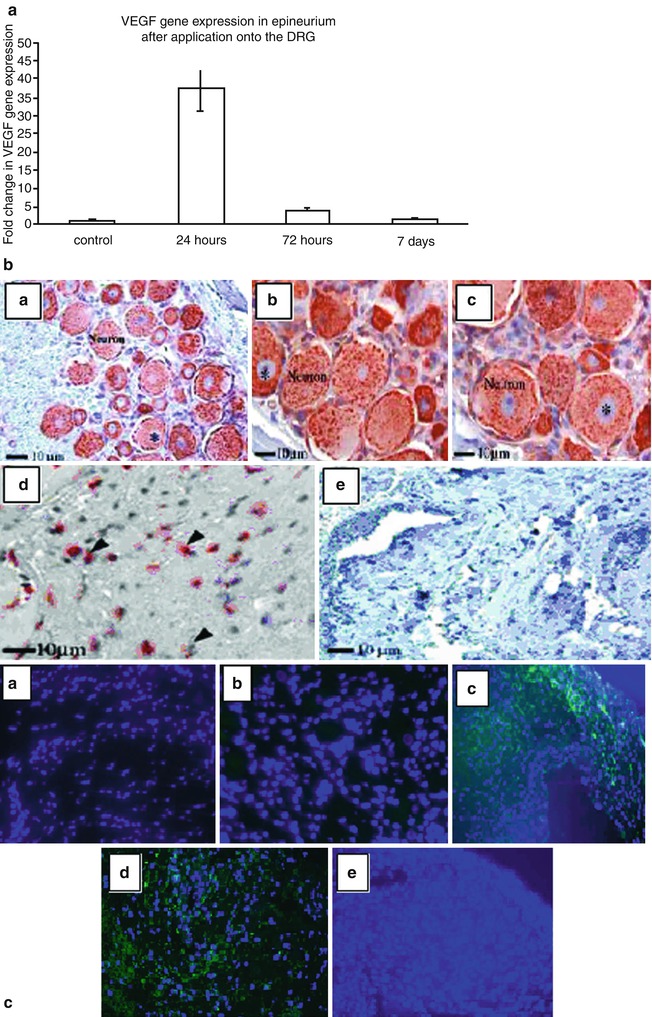

Fig. 61.2
VEGF analysis in naïve epineurium and epineurium after application onto the DRG. The level of VEGF gene expression assessed by Taqman real-time PCR in naïve epineurial sheath (control) and epineurial sheath after 24, 72 h and 7 days after application over the DRG (a). Due to the small size of samples epineural sheaths were harvested from six animals per time point and samples were pooled to obtain n = 2. Histoimmunoexpression of VEGF in DRG is depicted in b (a–d). Uniform and high expression of VEGF (brown color) was observed in naïve DRG neurons (a), 72 h after application of epineural sheath (b), and in contralateral undisturbed DRG (c). Epineural sheath after 72 h of topical application onto DRG also had very high expression of VEGF (d). Naïve control epineurium did not express any VEGF (e). Star indicates nucleai. Based on fluorescent staining no VEGF positive cells were present in control, undisturbed DRG (c, a). Group I with laminectomy only had a very weak expression of VEGF (c, b). High amount of VEGF positive cells were observed 24 and 72 h after application of epineural sheath onto the DRG in group II (c, c, d). No VEGF expression was detected 7 days after application of epineural sheath over DRG (c, e). Blue color (DAPI) represents nucleai, green color (FITC) represents VEGF positive cells. Pictures were taken under 40× magnification
Another key component expressed by the epineural sheath was laminin. Laminin is known to play an important role in neuroregeneration and it is one of the main glycoproteins of the basement membrane, it has been shown to participate in neuronal development, survival, and regeneration [19, 20]. It plays an essential role in vascular tissue organization, wound healing and supports the mechanical properties of vessels [21]. Using a cryo-immunogold technique Little et al. reported the absence of laminin from most of the collagen structures of the peripheral nerve [22]. Laminin labeling was found over the basal lamina and possibly over the immediately subjacent Schwann cell plasma membrane, but specific labeling appeared to be absent from other membranes (including those of non-myelinated axon/Schwann cell clusters) and from endoneurial collagen fibrils. The epineural sheath was not isolated in this case. Other authors discarded the epineural sheath as part of their experimental procedure or did not mention the epineurium in their results [23, 24]. This is in contrast to the findings in our study, where laminin was found throughout the epineurium. We hypothesized, that the presence of laminin in the epineural sheath can stimulate neuronal regeneration. Despite the importance of epinurial sheath in the context of peripheral nerve function, using a cryo-immunogold technique Little et al. reported the absence of laminin from most of the collagen structures of the peripheral nerve [22]. To date, there are no reported studies that characterize immunological and neuroregenerative features of the epineural sheath.
It is well known that the neo-vascularization is accompanied by increased infiltration of inflammatory cells [25, 26]. We found that when the epineural patch was applied to the DRG a robust increase in VEGF expression was observed. In addition to an increase in VEGF expression we also found a mild increase in the inflammatory cell infiltration as demonstrated by and increase in CD45 and CD3. This inflammatory response correlated with the amount of VEGF expression and decreased as VEGF expression declined at later time points.
One possible protective mechanism of epineurium can be related to its ability to modulate VEGF expression and angiogenesis and, as a consequence, regulate the inflammatory response with a potentially beneficial effects on scar formation. There was minimal CD45 and CD3 presence at 72 h and no presence at day 7 signifying a short lived response, perhaps mitigated by the epineurium.
VEGF over-expression has been shown to have a detrimental effect in the retina and it may contribute both to diabetic retinopathy and to neuropathy via the PKC pathway [27, 28]. Retinal hypoxia in the diabetic state due to reduction of blood flow stimulates the expression of VEGF and results in retinal neovascularization and increased retinal vascular permeability. Macular edema, bleeding, fibrosis, and loss of vision may follow. Local and systemic VEGF antagonists have been proposed as potential therapeutic interventions for the treatment of diabetic macular edema and proliferative retinopathy [29]. Conversely, treatment with exogenous VEGF may well exacerbate these disorders, and its use in clinical trials has been associated with the development of peripheral edema. This increase permeability could also lead to DRG fibrosis. The level of VEGF expression necessary for beneficial effects remains unknown but a nonlinear, dose-related, in vivo angiogenic effect of VEGF has been previously reported following intraperitoneal injection of a recombinant human VEGF (rhVEGF) saline solution in a rat model [30]. Recent studies have indicated that VEGF has direct neurotrophic effects on the central and peripheral nervous systems. VEGF stimulates axonal outgrowth and enhances the survival of superior cervical and DRG neurons, promotes neurogenesis in vitro and in vivo, and improves neuronal function and decreases degeneration after experimental spinal cord injury [31–33]. These finding were more evident at dose of 500 ng/ml rhVEGF compared to 700 ng/ml.
The ability of epineural sheath to serve as a reservoir of VEGF can be beneficial and facilitate neural regeneration. We showed that clinically apparent angioneogenesis occurs in the epineural sheath at the early stages after topical application onto the DRG. Increased perfusion of VEGF at this time point through the vasa nervorum to the chronically compressed neural elements could potentially augment neural regeneration. Based on our model the use of naturally occurring epineural sheath can provide the optimum reservoir of naturally expressed VEGF, with maximal levels reached 24 h after application onto DRG. Thus, the epineural sheath can play a dual role, as a reservoir of VEGF and a as a mechanical biologic barrier to diffusion. The sheath can be thought of as a scaffold that promotes angiogenesis, which has the potential to restore blood supply to chronically compressed and ischemic neural structures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








