Symptomatic criteria
Twelve (12) weeks or longer of two or more of the following:
Mucopurulent drainage
Nasal congestion
Facial pain, pressure, and fullness
Decreased sense of smell
And objective documentation by one or more of the following:
Endoscopic criteria
Purulent (not clear) mucous or edema in the middle meatus or ethmoid region
Polyps in nasal cavity or the middle meatus
Radiologic criteria
Radiographic imaging showing inflammation of the paranasal sinuses
Individually, patient’s symptoms can be nasal obstruction, congestion, purulent nasal discharge, and hyposmia which are found in an outstanding number of cases. Thus, the sensitivity for diagnosing CRS based on symptoms is very high, but the high prevalence of these symptoms results in a low specificity [6]. Consequently, documented physical exam findings or inflammation on imaging is required in addition to persistence of symptoms.
CT Scan
CT imaging is currently the gold standard for documenting paranasal sinus inflammation. CT scans accurately demonstrate the anatomy of the sinuses and clearly show mucosal thickening associated with the inflammatory state (see Fig. 3.1a, b).
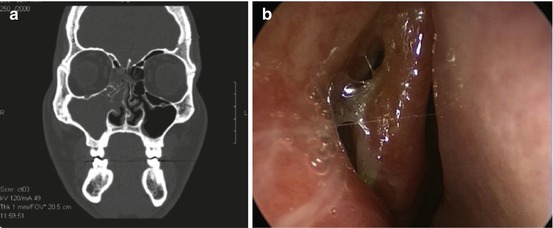

Fig. 3.1
(a) CT scan demonstrating maxillary and ethmoid opacification. (b) Endoscopy finding of bulging right maxillary sinus. This is the endoscopic visualization of the CT finding from (a) and demonstrates the role of endoscopy. In some cases endoscopy may reveal the same pathology but without associated radiation and cost
This inflammatory state may be graded using one of several systems, the progenitor of which and most commonly applied is the Lund-Mackay. In the Lund-Mackay system, individual sinuses and osteomeatal complexes are evaluated for inflammation. If inflammation occupies 0 % of a sinus on CT, a score of 0 is assigned; a maximum score of 2 is assigned when inflammation occupies 100 % of the sinus on CT. All other degrees of inflammation are scored as 1. Subsites include bilateral maxillary, ethmoid, sphenoid, frontal, and osteomeatal complexes. Scores are summed for a graded total of 0–24. This scoring allows for efficient and reliable total CT comparison [7, 8].
Although the scoring system is reliable, there are concerns over CT scans yielding false positives, and there is controversy surrounding the corroboration of imaging with the patient’s perception of symptoms. Henceforth, CT imaging has not been recommended as the sole diagnostic tool.
Supporting this negative assertion, Flinn evaluated CT scans of 100 patients and observed sinus opacification in 27 % of patients scanned for unrelated findings [9]. This figure and its implications on specificity for the diagnosis of CRS effectively argue against CT scan being the sole diagnostic tool.
Flinn’s conclusions were refuted by Calhoun et.al. in a subsequent study of 182 CT scan images [10]. He surmised that although CT scans of patients without symptoms of sinus disease do show some incidental sinus abnormalities, the incidence of such abnormalities is significantly greater in patients with a history of “sinus-type symptoms.” The apparently contradictory implications of Flinn’s and Calhoun’s conclusions demand a more complete diagnostic algorithm for a CRS diagnosis than a CT scan may be able to deliver alone.
Furthermore, with regard to corroboration of symptoms and CT scan findings, Bhattarcharyya et al. completed an exhaustive study with 586 patients. He concluded there was no observed link between severity of symptoms and degree of inflammation on CT imaging because patients who presented with facial pain or pressure were as likely to have abnormal results on CT scan as patients who presented without these symptoms [11].
In contrast to Bhatacharyya’s conclusions, Kenny et al. asserted that there was a correlation between CT scan and severity of other sinus symptoms. Prospective analysis of 304 patients demonstrated a link of the severity of CT scan and the severity of reported fatigue, sleep disturbance, nasal discharge or postnasal drip, nasal blockage, and decreased sense of smell [12].
Analysis of the aforementioned studies will show that while the data does not conflict, the conclusions do. This derived ambiguity ultimately supports the necessity for another tool besides CT imaging for the diagnosis of many cases of suspected CRS.
Nasal Endoscopy
Nasal endoscopy allows direct visualization of the entire nasal cavity and nasopharynx. This complete visualization may allow for the diagnosis of CRS in patients with sinonasal symptoms and nasal polyps (see Fig. 3.2) or purulent mucus in the middle meatus (see Fig. 3.3) or ethmoid region. As these findings may be subjective or not present at all, endoscopy is typically used to support a diagnosis of CRS, rather than define it. Alternately, it can be stated that the specificity of endoscopy demonstrates its utility, but the sensitivity of endoscopy limits its utility.
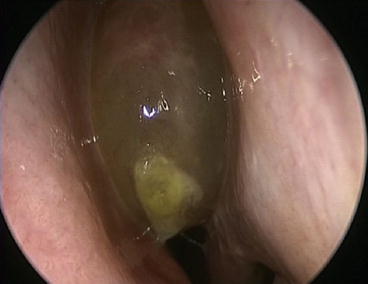
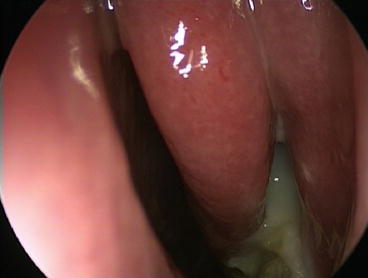

Fig. 3.2
Obstructive nasal polyp

Fig. 3.3
Purulence in the left middle meatus
There have been multiple studies investigating the role of endoscopy in the diagnosis of CRS compared to CT imaging. The specificity of these investigative tools has found to be similar. Casiano found that clinical staging of CRS based on endoscopy findings was as predictive of CT staging of CRS (see Fig. 3.1b). The author determined the overall specificity of endoscopy for the diagnosis of CRS to be 84 % [13]. Stankiewicz et al. corroborated these findings, noting that nasal endoscopy had a specificity of 86 % [14].
Bhattacharyya evaluated patient symptoms and endoscopic findings compared to CT findings. In patients meeting the symptom criteria for CRS, the sensitivity of symptoms alone was 88–96 %, but specificity was abysmal with a range of 9–12 %. However, when a positive endoscopic finding was added, specificity rose to a more acceptable 84 %. Even more interesting, for those patients not meeting the symptom guideline criteria, a positive finding on endoscopy had a specificity of 90 %, although this was not statistically significant [15]. These results placed emphasis on the role of nasal endoscopy in the accurate diagnosis of CRS.
Several otolaryngology researchers critiqued the conclusive support given to the specificity of endoscopy, arguing that positive findings on nasal endoscopy were flawed given the subjective interpretation required. Annamalai’s study in 2004 revealed that inter-rater agreement of mucosal edema was only “moderate” when compared to “very good” agreement for presence of discharge or polyps [16]. Annamalai suggested that polyps and purulence are clear physical findings, but mucosal changes require some subjective interpretation and therefore were less reliable as a diagnostic criterion.
Support for Annamalai’s findings was provided by two separate studies conducted by Raithatha and McCoul independently. Both found calculated inter-rater reliability was strong for gross abnormalities such as atypical lesions or polyps (see Fig. 3.4) but much weaker for more nuanced findings such as mucosal changes or middle turbinate obstruction [17, 18].
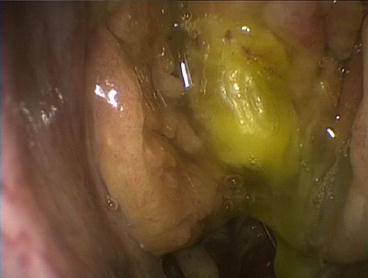

Fig. 3.4
Gross lesions such as polyps with eosinophilic mucin have high inter-rater agreement
The aforementioned conclusions are consistent with reports detailing the limited sensitivity (Table 3.2) [19] of nasoendoscopy. Ultimately, for those patients meeting symptom criteria but having negative endoscopic findings, a CT scan should be considered if clinical suspicion for CRS is high. Despite its limits, the role of endoscopy has been recognized [6]. Patients that meet symptom criteria of CRS and have polyps or purulent mucosa may be confidently diagnosed with endoscopy without the use of CT imaging.
Table 3.2
Sensitivity and specificity of nasal endoscopy
Bhattacharyya | Stankiewicz | Agius | |
|---|---|---|---|
Sensitivity | 0.46 | 0.46 | .91 or .51 |
Specificity | 0.84 | 0.86 | 0.71 |
Positive predictive value | 0.66 | 0.74 | 0.62 |
Negative predictive value | 0.7 | 0.64 | 0.71 |
Procedure
Effective clinical use of nasal endoscopes requires familiarity with the technology, knowledge of its limitations, expanded applications, and technical training in procedural methods. Technologic familiarity should encompass critical evaluation of strengths and weaknesses of rigid and fixed endoscopes, angulation of endoscopic lenses, and diameter of endoscopic rods. An astute clinician will be able to select appropriate instrumentation for most cases with knowledge and experience with these categories.
Furthermore, an endoscopist will understand that instrument use may exceed diagnostic purposes. For example, endoscopy permits the physician to gauge response to treatment and prescribe culture-directed antibiotics. Additionally, the use of endoscopy in teaching should not be understated, as it has a clear role in patient and resident education.
Like any procedure, technical training is essential, and contraindications though rare are present. Awareness and prophylactic mitigation of potential complications such as epistaxis or vasovagal response may ensure a successful exam. The generated exam findings are most useful when used in documented endoscopic staging systems.
Equipment
As with most procedures, endoscopy has a broad set of equipment with equally broad applications. There are utilitarian devices (i.e., 0° rigid 4.0 mm endoscope) with features that may be useful in most settings, and there are specialized instruments (i.e., 70° endoscopes, flexible endoscopes) that are more useful for obtaining optimal results in patients with altered anatomy. Rigidity, diameter, and lens angulation are the defining criteria of endoscope technology. Thus knowledge of categorical advantages and disadvantages are necessary for endoscope selection.
Rigid and Flexible Endoscopes
Rigid endoscopes are available in multiple diameters (2.7–4 mm) and divergent angles (0–70°). By virtue of their protected optic fibers and increased diameter, these endoscopes provide superior clarity and magnification allowing easy visualization of signs of chronic rhinosinusitis. Rigid endoscopy may be performed with one hand thereby allowing the clinician to perform procedures such as debridement, biopsies, and polypectomies. Furthermore, it has been posited that clinical examination with the rigid endoscope familiarizes trainees with its use for endoscopic sinus surgery. Some have suggested that rigid endoscopes may prove uncomfortable for patients [20], while others have shown that patients feel more discomfort with flexible scopes [21].
Alternatively, flexible endoscopes may be used to evaluate the nasal cavity. Their thin, flexible body and tip can be angulated up or down to help maneuver the scope. Although these maneuvers help visualize areas that cannot be seen with a rigid endoscope, such as the floor of the maxillary sinuses or superior aspects of the frontal sinuses, they may not be useful in all situations. This was confirmed in a prospective randomized study by Midwinter et al. who found that rigid endoscopes allow for increased visualization of most sinonasal structures, the exception being the nasopharynx [21]. Thus most patients benefit from rigid endoscopic examination, but those with obstructing anatomy or concurrent laryngeal/pharyngeal complaints may benefit from nasal examination with a flexible fiber-optic endoscope.
An unavoidable major disadvantage of flexible endoscopes is the decreased quality of images necessitated by the bending of optic fibers and necessary limits of diameter demanded by mobility. Also, patients may find the sensation of a moving tip uncomfortable [21]. Furthermore, using the flexible scope eliminates the ability to perform two-handed techniques such as nasal cavity debridement following sinus surgery. The increased cost of flexible endoscopes can also be a deterrent to their use [21].
Diameter
Large-diameter endoscopes (4.0 mm) provide a wide panoramic view with excellent clarity and magnification and the resulting ability to ascertain subtle rhinologic pathology. These qualities ensure that the 4.0 mm scope is the benchmark endoscopic examination tool for adults. Disadvantages of large-diameter endoscopes include the possibility of patient discomfort, a higher risk of mucosal trauma, and inability to view pathology in patients with anatomic abnormalities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






