Disorders of the Eccrine Sweat Glands and Sweating: Introduction
|
Disorders of eccrine sweating can occur for many different reasons, including dysfunction of the thermoregulatory centers in the brain’s central autonomic network, changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons/axons or in the muscarinic (M3) cholinergic synapse on sweat glands. Abnormalities of eccrine sweat formation by the secretory coil and sweat ductal cells may occur or ductal disruption or occlusion may develop, preventing delivery of sweat to the skin surface.
A review of the normal anatomy and physiology of eccrine sweat glands and sweating may be found in Chapter 83. This chapter focuses on neurologic and dermatologic disorders that cause focal or generalized abnormalities of sweating, highlighting an exciting interface where disorders are better understood, diagnosed, and treated based on recognition of the integrated function of nerves, skin, and the immune system (Fig. 84-1). There are a variety of techniques that can be used clinically and in research of sweating that are discussed online. Table 84-1 is a comprehensive table based on disorders with increased or decreased sweating—some diseases are discussed in this text–the others are discussed online. Many are disorders related to nervous system structural, functional and inherited disorders; therefore any patient with a sweating disorder should be evaluated carefully for internal medicine diseases and neurologically by consultants in some cases.
Figure 84-1
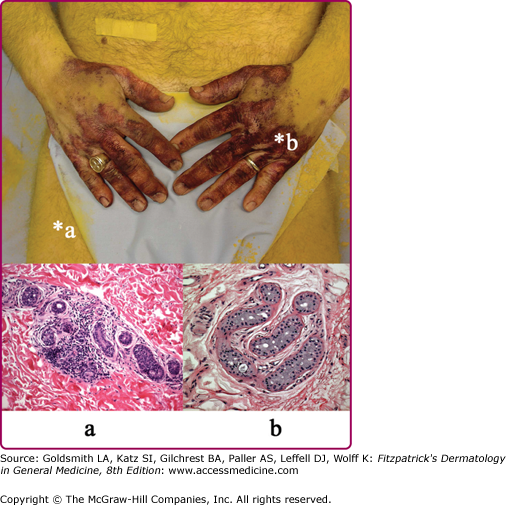
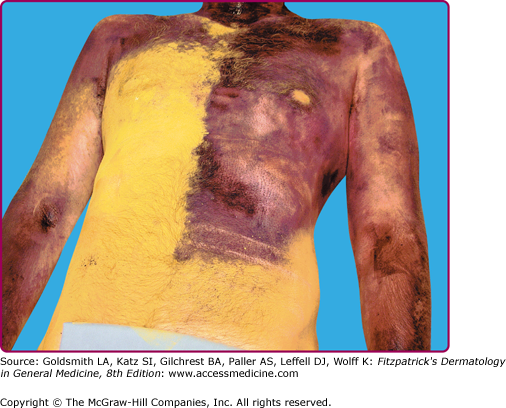
A patient with an acquired idiopathic anhidrosis shows anhidrotic (yellow) and sweating (purple) staining of sodium alizarin sulfonate (alizarin Red S) indicator powder. Punch skin biopsy from an anhidrotic skin site (a) shows marked perieccrine lymphocytic infiltration of sweat gland secretory coils, whereas sweating skin (b) shows normal sweat gland morphology. The presence of perieccrine inflammation prompted immunomodulatory therapy with corticosteroids and pulsed methotrexate and a trial of topical tacrolimus.
Primary Focal (Essential) Hyperhidrosis Palmoplantar, axillary, craniofacial, generalized hyperhidrosis Secondary Causes of Focal Hyperhidrosis Due to cerebral infarction
Associated with spinal cord injury
Associated with other central nervous system disorders
Associated with peripheral nervous system disorders
Associated with local skin disorders
| Secondary Causes of Generalized Hyperhidrosis Associated with central nervous system disorders
Associated with fever and chronic infection
Associated with metabolic and systemic medical diseases
Associated with malignancy
Medication-induced
Toxic syndromes
Associated with central and peripheral nervous system disorders
Primary Autonomic Disorders with Acquired Anhidrosis Isolated sudomotor disorders
Sudomotor plus other autonomic disorders
Secondary Autonomic Disorders Associated with Anhidrosis Central nervous system lesions (stroke, tumor, infection, infiltration, trauma, etc.)
Degenerative disorders
| Peripheral Nerve Lesions Causing Anhidrosis
Anhidrosis due to toxins, pharmacologic agents and heat exposure
Anhidrosis Associated with Diseases of Skin and Sweat Glands Anhidrosis due to physical agents damaging skin
Anhidrosis due to congenital and acquired skin diseases
Disorders affecting the sweat duct
Disorders with abnormal sweat composition
|
 In Vivo Methods of Studying Sweat Gland Function
In Vivo Methods of Studying Sweat Gland Function
![]() Sweat is readily visualized by a topical indicator such as iodinated starch1,2 or sodium alizarin sulfonate (alizarin Red S).3 These techniques are used to evaluate large body surfaces (Fig. 84-2). Iodinated starch powder is prepared by adding 0.5–1.0 g of iodine crystals to 500 g of soluble starch in a tightly capped bottle. Sodium alizarin sulfate is mixed with an equal amount (by weight) of sodium carbonate anhydrous and twice the amount of cornstarch. Both are applied to the skin and undergo a dramatic color change when moistened by the water (sweat) from activated sweat glands. For smaller body areas, a Minor’s starch-iodine test for excessive sweating may be performed by applying betadine on Q-tips to the affected area. Once the iodine solution has dried, cornstarch can be lightly sprinkled on the area. Deep purple color change will develop over time in the areas affected with hyperhidrosis.
Sweat is readily visualized by a topical indicator such as iodinated starch1,2 or sodium alizarin sulfonate (alizarin Red S).3 These techniques are used to evaluate large body surfaces (Fig. 84-2). Iodinated starch powder is prepared by adding 0.5–1.0 g of iodine crystals to 500 g of soluble starch in a tightly capped bottle. Sodium alizarin sulfate is mixed with an equal amount (by weight) of sodium carbonate anhydrous and twice the amount of cornstarch. Both are applied to the skin and undergo a dramatic color change when moistened by the water (sweat) from activated sweat glands. For smaller body areas, a Minor’s starch-iodine test for excessive sweating may be performed by applying betadine on Q-tips to the affected area. Once the iodine solution has dried, cornstarch can be lightly sprinkled on the area. Deep purple color change will develop over time in the areas affected with hyperhidrosis.
![]() Sweat gland activity can be studied quantitatively by a number of techniques including: filter paper collection, weighing and analyzing of sweat, the quantitative sudomotor axon reflex test (QSART),4 skin sympathetic potentials,5 silastic mold or iodine-impregnated paper imprint after pilocarpine stimulation,6 microcannulation of the sweat duct or coil,7 collection into a Wescor–Macroduct coil (Logan, CT),8 humidity sensors within ventilated capsules measuring transepidermal water loss,9,10 and by determining total body surface anhidrosis to a maximal thermoregulatory stimulus during a thermoregulatory sweat test (TST).11,12 Other, more sophisticated techniques use microdialysis membranes delivering minute quantities of transmitter substances to the dermis13 or use confocal electron microscopy and immunohistochemical analysis of biopsied skin stained for peptides and proteins that comprise the structure and innervation of the sweat gland14 (Fig. 84-3).
Sweat gland activity can be studied quantitatively by a number of techniques including: filter paper collection, weighing and analyzing of sweat, the quantitative sudomotor axon reflex test (QSART),4 skin sympathetic potentials,5 silastic mold or iodine-impregnated paper imprint after pilocarpine stimulation,6 microcannulation of the sweat duct or coil,7 collection into a Wescor–Macroduct coil (Logan, CT),8 humidity sensors within ventilated capsules measuring transepidermal water loss,9,10 and by determining total body surface anhidrosis to a maximal thermoregulatory stimulus during a thermoregulatory sweat test (TST).11,12 Other, more sophisticated techniques use microdialysis membranes delivering minute quantities of transmitter substances to the dermis13 or use confocal electron microscopy and immunohistochemical analysis of biopsied skin stained for peptides and proteins that comprise the structure and innervation of the sweat gland14 (Fig. 84-3).
Figure 84-3
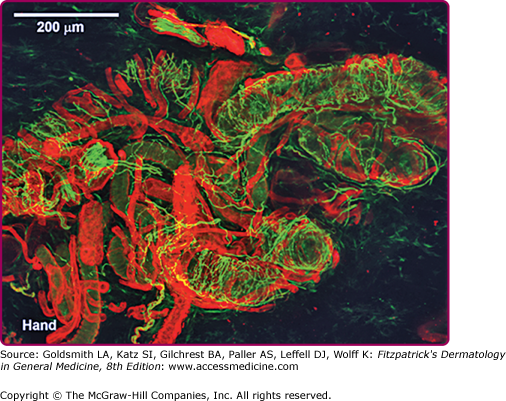
Secretory tubules, nerve fibers, and blood vessels in a normal human sweat gland fluorescently stained by immunohistochemical and lectin methods and visualized via laser scanning confocal microscope. Immunoreactive staining of nerves with protein gene product 9.5 antibody (green staining) shows axons wrapped around secretory tubules. (Photo used with permission from Dr. William Kennedy, MD, Minneapolis, MN.)
![]() It is desirable to combine several methods for determining the integrity of the eccrine sweat response. For example, a TST can be combined with tests of the sweat gland and/or its peripheral nerve innervation to localize a sweating disorder to the peripheral or central nervous system. Alternatively, a volumetric technique can be combined with a sweat droplet distribution imprint to estimate the sweat volume per active gland.
It is desirable to combine several methods for determining the integrity of the eccrine sweat response. For example, a TST can be combined with tests of the sweat gland and/or its peripheral nerve innervation to localize a sweating disorder to the peripheral or central nervous system. Alternatively, a volumetric technique can be combined with a sweat droplet distribution imprint to estimate the sweat volume per active gland.
![]() The composition of collected sweat can give critical information about eccrine function. For example, sweat chloride ion concentration can determine the integrity of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel and provide diagnostic information for cystic fibrosis.15
The composition of collected sweat can give critical information about eccrine function. For example, sweat chloride ion concentration can determine the integrity of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel and provide diagnostic information for cystic fibrosis.15
Visual Examination of the Skin
The physical examination continues to be important in the diagnosis of sweating disorders. For example, the skin of an infant with cystic fibrosis is much saltier than infants without cystic fibrosis and when collected sweat is dried, it forms fern-like crystals. Infants with widespread sweat loss may present with “fever of unknown origin.” Taking note of the beads of sweat on the palms and soles of a young adult goes a long way to making a diagnosis of primary focal (essential) hyperhidrosis. In uremia, the evaporation of sweat with high urea concentrations results in the deposition of urea on the skin, referred to as uremic frost. Patients with craniofacial hyperhidrosis may develop a darkened hue to their skin known as chromhidrosis. Focal or segmental compensatory hyperhidrosis may be apparent on examination. Dry, atrophic skin may be seen in areas affected by small-fiber neuropathy. Disorders affecting the sweat duct (miliaria or psoriasis, for example) are often diagnosed via visual inspection of the skin.
Disorders of Sweating
Primary Focal (Essential) Hyperhidrosis
Primary focal (essential) hyperhidrosis is one of the most common disorders of eccrine sweating.16 This condition is defined as focal visible, excessive sweating of at least 6-months’ duration without apparent cause with at least two of the following characteristics: (1) bilateral and relatively symmetric sweating; (2) the sweating impairs daily activities; (3) a frequency of at least one episode per week; (4) an age of onset before the age of 25 years; (5) a positive family history; (6) cessation of sweating during sleep.17 A national survey estimated that hyperhidrosis affects 2.8% of the US population, and that half of those affected experience excessive sweating of the axillae.18 Hyperhidrosis of the palms, soles, axillae, and, to a lesser extent, craniofacial and groin regions may occur at any time irrespective of temperature, stress, or pleasure. Primary focal hyperhidrosis does not manifest when the patient undergoes general anesthesia and augmentation with thermal stimuli and physical exertion is not uncommon. Sweating may be continuous or phasic; when continuous, sweating is most troublesome in summer. Phasic outbursts with minor emotional activity are similar year round.
Severe primary focal hyperhidrosis interferes with many activities of daily living and patients report a reduced quality of life.19 Avoiding a handshake can lead to professional embarrassment, and avoidance of touch can lead to social or interpersonal seclusion and other symptoms of social anxiety disorder. Symptoms begin in childhood or around puberty and affect either sex equally. A family history is present in approximately one-fourth of patients. The disorder persists for years with occasional spontaneous improvement after 35 years of age.
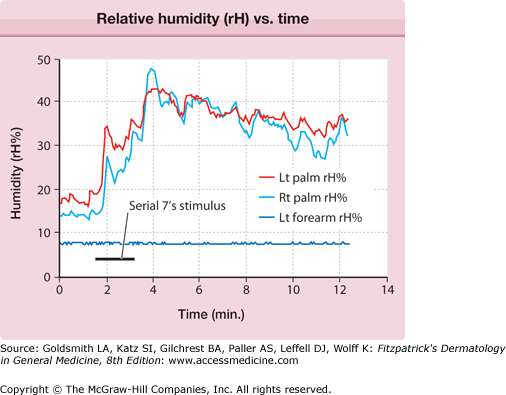
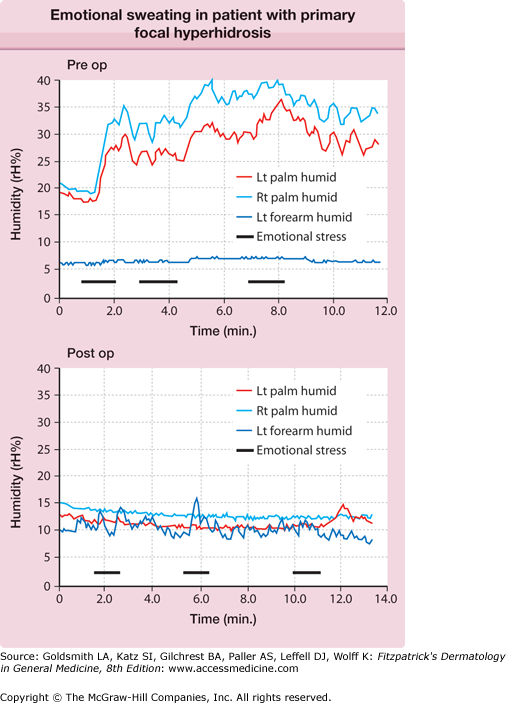
The central autonomic network control of emotional sweating is distinct from the preoptic-anterior hypothalamic controller of thermoregulatory sweating. The anterior cingulate cortex, which orchestrates the sweat response from the palms and soles, may modulate hypothalamic output inappropriately. These patients have less reflex bradycardia than control subjects in response to the Valsalva maneuver or facial immersion, but a higher degree of cutaneous vasoconstriction in response to finger immersion in cold, suggesting that they have increased sympathetic outflow passing through the T2–T3 ganglia.20 Ventilated capsule recordings of palmar sweating provide further evidence of emotionally triggered, centrally derived sweat output. Such sweating is augmented at rest, bilaterally synchronous, and pulsatile (eFig. 84-3.1).
eFigure 84-3.1
Ventilated capsule recording of relative humidity (rH) versus time. Bilateral palmar and left (Lt) forearm quantitative emotional sweat response from subject with primary focal hyperhidrosis. Mental arithmetic (serial subtractions from 100 by 7) triggered vigorous sweating. Note the synchrony between left and right (Rt) palm, the pulsatility of the response, and the anatomic confinement of the response to the palms, all characteristic of this disorder.
The severity of primary focal hyperhidrosis ranges from intermittent, slightly moist palms and soles to daily sweat drippage from hands and feet, requiring the frequent use of towels.
Treatment involves determining the severity and distribution of the disorder, ruling out other causes of hyperhidrosis, and selecting a modality of therapy appropriate to age of the patient and severity of the disorder. Some current treatment methods are shown in Box 84-1. Treatment begins with discussing the nature of the disorder and discerning patient wishes and expectations. Mild cases of axillary and palmar sweating are controlled via topical application of aluminum chloride hexahydrate or glycopyrrolate, an anticholinergic agent. Care should be taken to have the aluminum chloride applied to completely dry skin as use of the agent on moist skin can result in the formation of a weak hydrochloric acid that can result in discomfort, and burning and peeling of the skin surface. This irritation can be managed by cessation of therapy for a few days and the use of a class VII topical steroid cream. Oral glycopyrrolate, oral or transdermal clonidine, transdermal scopolamine, or oral topiramate21 can be administered if topical agents alone are not helpful or are too irritating. Control of palmar-plantar sweating is sometimes possible using tap water (or a solution of glycopyrrolate) iontophoresis. Intradermal injection of botulinum toxin (BTX), (onabotulinumtoxinA) is appropriate for treatment of focal areas, such as axillae, palms of hands, soles of feet and groin, and is effective for 2–8 months per treatment.22 Facial hyperhidrosis can be treated with oral or topical23 glycopyrrolate.
Type of Hyperhidrosis | Treatment | Formulation | Route of Administration and Dosage |
|---|---|---|---|
Craniofacial, gustatory | Glycopyrrolate | 0.5%–2% Vanicream base or roll-on lotion; 1- to 2-mg tablet | Topical, once to twice daily. Tablet started at 1 mg bid; increase prn up to 2 mg three to four times daily. |
Clonidine | 0.1-mg tablet, 0.1–0.3 mg/day, transdermal patch | Oral incremental increases up to 0.6–1.2 mg/day in two or three divided doses; 0.1 mg/day patch first week increased weekly up to two 0.3 mg/day patches. | |
Craniofacial, gustatory Paroxysmal localized | Donnatal extend tab | Phenobarbital, USP (3/4 gr.) 48.6 mg Hyoscyamine Sulfate, USP 0.3111 mg Atropine Sulfate, USP 0.0582 mg Scopolamine Hydrobromide, USP 0.0195 mg | One tablet orally every 12 hr. |
Topiramate | 25-mg tablet | Begin 25 mg twice daily; increase each dose weekly by 25 mg up to 100–200 mg daily in two divided doses. | |
Axillary, palmar-plantar, craniofacial | Aluminum chloride | 20% aluminum chloride in anhydrous ethyl alcohol; 12% aluminum chloride in sodium carbonate water | Topically, nightly, until desired sweat reduction achieved. Then taper to once weekly; follow Physicians’ Desk Reference directions carefully for each body region to maximize effect and minimize skin irritation. |
Axillary | Over the counter clinical strength antiperspirant | Aluminum Zirconium Trichlorohydrex Gly 20% anhydrous | Apply to axillae twice a day |
Palmar–plantar | Iontophoresis unit | Patient-controlled current, 15–30 mA using tap water; glycopyrrolate can be added, making a 0.1% solution | Immerse hands, feet for 30 min once or twice daily, or 20 min at each site every 2–3 days, or 10 min at each site three to five times weekly; anode site is most effective so switch sides after one-half of each treatment; direct current more effective, but AC current less painful and may be used part of time; glycopyrrolate solution enhances effect. Most modern iontophoresis unit do not require switching of anodes. |
Palmar–plantar, axillary, gustatory, recalcitrant | Botulinum toxin A injection | 2–5 units of botulinum toxin | Given via high intradermal injections to ∼1 cm2 iced/anesthetized skin areas; for botulinum toxin A, total of 12–20 injections (50 units total) given per axilla, 50–75 injections in the palm/each foot; benefit lasts 2–8 months; reinjection is effective. |
Glycopyrrolate | 1- to 2-mg tablet | Tablet started at 1 mg bid; increase 1 mg each week as tolerated up to 2 mg three to four times daily. | |
Axillary | Local incision | Liposuction and curettage of sweat glands in dermis | Define area of involvement with starch-iodide Minor test or equivalent; small incisions with sharp suction-curettage cannula; usually permanent, 40%–70% reduction in sweating achieved. |
Palmar, axillary; documented severely affected cases resistant to other treatment modalities | Sympathetic surgery | Various procedures affecting the T2, T3, and/or T4 sympathetic ganglia and connections | Invasive, video-assisted endoscopic thoracic procedure preferred; intraoperative monitoring of hand/finger skin blood flow/temperature suggested; sympathotomy (disconnection of sympathetic chain between T2 and stellate ganglia done to minimize compensatory hyperhidrosis; selective T3 sympathectomy may have less compensatory hyperhidrosis; T2–T4 sympathectomy done for axillary hyperhidrosis has high incidence of compensatory hyperhidrosis on trunk and gustatory hyperhidrosis. |
Immunohistochemical studies show sweat glands appear structurally normal in hyperhidrotic patients before BTX therapy, whereas after therapy the luminal area of the gland and protein gene product 9.5 (neural marker) staining is diminished. This suggests that BTX therapy induces long-standing functional denervation of the sweat glands. However, growth-associated protein 43, indicative of axonal sprouting, is increased, suggesting reinnervation may eventually take place.24
Repeat BTX injections do not demonstrate a diminished duration of efficacy.25 Pain with palmar botulinum injections is common and several techniques are in use to minimize this.26,27 Iontophoresis of BTX in solution has been successfully tried with better control of sweating than with water alone.28,29 However, the cost effectiveness of this has been questioned.
Only the most severe cases of palmar hyperhidrosis not responding to conservative treatment may be considered for surgical management. Many patients have been successfully treated with endoscopic, upper thoracic sympathectomy30 (eFig. 84-3.2); however, the best procedure to use is still being investigated.10,30–36 These surgical procedures carry the risk of creating minor to severe compensatory hyperhidrosis in body segments below the treated area (see eFig. 84-3.3) as well as much less common surgical complications of wound infection, Horner’s syndrome, pneumo- or hemothorax, and atelectasis. Long-term surveys of patients having undergone thoracic sympathectomy for treatment of palmar hyperhidrosis have illustrated that many patients wish they had not undergone the procedure due to the compensatory sweating that developed after the surgery.37,38
eFigure 84-3.2
A. Preoperative (PRE OP) emotional sweating in patient with primary focal hyperhidrosis, B. The emotional sweat response has been eliminated by a second thoracic (T2) endoscopic thoracic sympathotomy. Quantitative emotional sweating measured via ventilated capsule-relative humidity recording. Lt = left; POST OP = postoperatively; Rt = right. Source: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ: Fitzpatrick’s Dermatology in General Medicine, 7th Edition:
eFigure 84-3.3
Truncal compensatory hyperhidrosis in a patient 4 months after bilateral, T2, endoscopic sympathotomy for primary focal hyperhidrosis of palms. Sodium alizarin sulfate indicator shows sweating areas in purple color; patient imaged during the postoperative thermoregulatory sweattest. Source: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ: Fitzpatrick’s Dermatology in General Medicine, 7th Edition:
Another surgical option for axillary hyperhidrosis is tumescent suction curettage, which is performed in an outpatient setting. In one limited study of 63 subjects, greater than 75% were still satisfied with their reduction in sweating 2 years after treatment.39
(Secondary causes of localized hyperhidrosis are discussed in the next section.)
Secondary Causes of Localized Hyperhidrosis
![]() Hemispheric strokes, especially those affecting the insular and opercular cortex, can produce contralateral hemihyperhidrosis primarily affecting the face and upper extremities. Unilateral hypothalamic, peduncular, pontine, and medullary infarcts can do the same, even in the absence of ipsilateral Horner syndrome. Bilateral pontine and cerebellar infarction can produce facial hyperhidrosis. The hyperhidrosis is acute and transient. Putative mechanisms involve interruption of inhibitory pathways controlling contralateral sweating.
Hemispheric strokes, especially those affecting the insular and opercular cortex, can produce contralateral hemihyperhidrosis primarily affecting the face and upper extremities. Unilateral hypothalamic, peduncular, pontine, and medullary infarcts can do the same, even in the absence of ipsilateral Horner syndrome. Bilateral pontine and cerebellar infarction can produce facial hyperhidrosis. The hyperhidrosis is acute and transient. Putative mechanisms involve interruption of inhibitory pathways controlling contralateral sweating.
![]() Patients with spinal cord injuries experience episodes of profuse sweating weeks, months, or years after injury. The areas of hyperhidrosis are determined by the complex interaction of exaggerated somatosympathetic reflexes, and the longitudinal and transverse extent, level, and completeness of the cord lesion. The lowest segment of cord injury is usually above T6 and the segmental hyperhidrosis involves the face, neck, and upper trunk most commonly. Associated symptoms include facial flushing and nasal congestion, headaches, piloerection, hypertension, and bradycardia. Stimuli, such as distention of the bowel or bladder, skin and visceral organ inflammation, and orthostatic hypotension, stimulate uninhibited sympathetic preganglionic neurons. Removing the stimulus is the most effective treatment, although antihypertensives (clonidine, α blockers, and calcium channel blockers) and anticholinergic agents (propantheline, glycopyrrolate) are sometimes needed.
Patients with spinal cord injuries experience episodes of profuse sweating weeks, months, or years after injury. The areas of hyperhidrosis are determined by the complex interaction of exaggerated somatosympathetic reflexes, and the longitudinal and transverse extent, level, and completeness of the cord lesion. The lowest segment of cord injury is usually above T6 and the segmental hyperhidrosis involves the face, neck, and upper trunk most commonly. Associated symptoms include facial flushing and nasal congestion, headaches, piloerection, hypertension, and bradycardia. Stimuli, such as distention of the bowel or bladder, skin and visceral organ inflammation, and orthostatic hypotension, stimulate uninhibited sympathetic preganglionic neurons. Removing the stimulus is the most effective treatment, although antihypertensives (clonidine, α blockers, and calcium channel blockers) and anticholinergic agents (propantheline, glycopyrrolate) are sometimes needed.
![]() Recent studies have shown that sprouting of afferent fibers in the dorsal horn of thoracolumbar cord segments below the lesion and severe necrosis of descending white matter tracts in the vicinity of the lesion are associated with autonomic dysreflexia. Novel treatments that inhibit nerve growth factor-induced sprouting and that suppress immune-mediated responses to tissue injury are emerging as therapeutic agents41–45 that promise to limit dysreflexia development.
Recent studies have shown that sprouting of afferent fibers in the dorsal horn of thoracolumbar cord segments below the lesion and severe necrosis of descending white matter tracts in the vicinity of the lesion are associated with autonomic dysreflexia. Novel treatments that inhibit nerve growth factor-induced sprouting and that suppress immune-mediated responses to tissue injury are emerging as therapeutic agents41–45 that promise to limit dysreflexia development.
![]() Spontaneous syringomyelia with Chiari’s I and II malformations and incomplete or asymmetric myelopathies may produce segmental hyperhidrosis not associated with other features of dysreflexia. These represent examples of compensatory or perilesional hyperhidrosis most times.
Spontaneous syringomyelia with Chiari’s I and II malformations and incomplete or asymmetric myelopathies may produce segmental hyperhidrosis not associated with other features of dysreflexia. These represent examples of compensatory or perilesional hyperhidrosis most times.
![]() Cold-induced sweating syndrome is a genetically heterogenous autosomal recessive condition associated with alterations of a composite cytokine, which is composed of cardiotrophin-like cytokine factor 1 (CLCF1) and cytokine receptor-like factor 1 (CRLF1).46 The cold-induced sweating involves the upper body segments, which paradoxically do not sweat in the heat. Cold-induced sweating is associated with limited autonomic failure, motor neuropathy, skeletal deformities, and abnormal facies that include a high arched palate. Long-term symptom control has been reported with a combination of low dose amitriptyline and clonidine.46 In olfactory hyperhidrosis syndrome,48 profuse facial sweating is precipitated by perfume smells, but not by gustatory or mental stimuli, and may respond to amitriptyline.
Cold-induced sweating syndrome is a genetically heterogenous autosomal recessive condition associated with alterations of a composite cytokine, which is composed of cardiotrophin-like cytokine factor 1 (CLCF1) and cytokine receptor-like factor 1 (CRLF1).46 The cold-induced sweating involves the upper body segments, which paradoxically do not sweat in the heat. Cold-induced sweating is associated with limited autonomic failure, motor neuropathy, skeletal deformities, and abnormal facies that include a high arched palate. Long-term symptom control has been reported with a combination of low dose amitriptyline and clonidine.46 In olfactory hyperhidrosis syndrome,48 profuse facial sweating is precipitated by perfume smells, but not by gustatory or mental stimuli, and may respond to amitriptyline.
![]() Abnormal thoracic sympathetic activity due to encroachment of mass lesions (Pancoast tumor, mesothelioma, lymphoma, osteoma, cervical rib) on the sympathetic trunk or postganglionic fibers can cause segmental hyperhidrosis.49 Sweating is usually spontaneous and located in the distribution of the involved sympathetic trunk or segmental spinal nerve root. An area of anhidrosis may be juxtaposed with the area of hyperhidrosis or the hyperhidrosis is contralateral to the lesion. Nonmalignant causes of the same phenomenon include diabetic and idiopathic immune-mediated truncal neuropathy (see eFig. 84-3.4).
Abnormal thoracic sympathetic activity due to encroachment of mass lesions (Pancoast tumor, mesothelioma, lymphoma, osteoma, cervical rib) on the sympathetic trunk or postganglionic fibers can cause segmental hyperhidrosis.49 Sweating is usually spontaneous and located in the distribution of the involved sympathetic trunk or segmental spinal nerve root. An area of anhidrosis may be juxtaposed with the area of hyperhidrosis or the hyperhidrosis is contralateral to the lesion. Nonmalignant causes of the same phenomenon include diabetic and idiopathic immune-mediated truncal neuropathy (see eFig. 84-3.4).
eFigure 84-3.4
Focal hyperhidrosis and anhidrosis due to truncal neuropathy. Anhidrosis (yellow color) depicts a left T3 and right T5 radiculopathy. Note dark purple staining area of perilesional hyperhidrosis (sodium alizarin sulfate indicator). Source: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ: Fitzpatrick’s Dermatology in General Medicine, 7th Edition: .
![]() Segmental or localized hyperhidrosis commonly occurs postsympathectomy and in the primary autonomic disorders such as pure autonomic failure (PAF) and Ross syndrome. The latter two disorders are discussed in Section “Primary Autonomic Disorders with Acquired Idiopathic Anhidrosis.”
Segmental or localized hyperhidrosis commonly occurs postsympathectomy and in the primary autonomic disorders such as pure autonomic failure (PAF) and Ross syndrome. The latter two disorders are discussed in Section “Primary Autonomic Disorders with Acquired Idiopathic Anhidrosis.”
Localized sweating on lips, forehead, scalp, and nose while eating hot and spicy foods occurs physiologically in many people via a trigeminovascular reflex. Pathologic gustatory hyperhidrosis is asymmetric, intense, and may produce patches of sweating on the trunk and even extremities. The cause is aberrant regeneration of damaged and undamaged facial parasympathetic fibers, destined for salivary glands, which instead supply facial sweat glands that have been sympathetically denervated. Thus, gustatory stimuli that previously caused parotid, salivary gland, or gastric secretion now also cause sweating in the distribution of the damaged sympathetic nerve. The most common occurrence is in Frey syndrome, in which sweating occurs in the distribution of the auriculotemporal nerve after an injury, abscess, or surgery in the parotid region.50 Frey syndrome can be seen in infants and children, often following birth trauma with forcep delivery, but two cases of familial, bilateral Frey syndrome without birth trauma have also been reported.51 Gustatory sweating may follow upper thoracic and cervical sympathectomy,50,52–54 facial herpes zoster, or chorda tympani injury and has been reported in cluster headache, diabetic neuropathy,55–57 encephalitis, syringomyelia, and invasion of the cervical sympathetic trunk by a tumor.58 The exact distribution can be delineated with indicator powder on the face, neck, and upper trunk while the subject chews and is photographed. Treatment with topical scopolamine, clonidine,53 glycopyrrolate,59,60 aluminum chloride, or BTX injection61,62 can be effective; rarely, intracranial section of the glossopharyngeal nerve or tympanic neurectomy is needed.
![]() Continuous profuse sweating in the medial supraorbital region associated with Raeder syndrome (Horner syndrome plus temporal and frontal headache and paresthesia) is known as lacrimal sweating. Sudomotor fibers to the medial forehead traveling with the internal carotid artery presumably are damaged and parasympathetic fibers to the lacrimal gland provide aberrant regeneration to the nearby denervated sweat glands.
Continuous profuse sweating in the medial supraorbital region associated with Raeder syndrome (Horner syndrome plus temporal and frontal headache and paresthesia) is known as lacrimal sweating. Sudomotor fibers to the medial forehead traveling with the internal carotid artery presumably are damaged and parasympathetic fibers to the lacrimal gland provide aberrant regeneration to the nearby denervated sweat glands.
![]() Harlequin syndrome (unilateral facial flushing and sweating with heat and exercise) may develop abruptly posttrauma or stroke or as an immune-mediated ganglionopathy. The flushed, sweating side attracts attention, but it is the contralateral side with anhidrosis and loss of flushing that has a sympathetic abnormality. Occasionally, tonic pupils may occur, forming an overlap with Ross syndrome.65,66
Harlequin syndrome (unilateral facial flushing and sweating with heat and exercise) may develop abruptly posttrauma or stroke or as an immune-mediated ganglionopathy. The flushed, sweating side attracts attention, but it is the contralateral side with anhidrosis and loss of flushing that has a sympathetic abnormality. Occasionally, tonic pupils may occur, forming an overlap with Ross syndrome.65,66
![]() The involved area in idiopathic unilateral circumscribed hyperhidrosis is usually sharply demarcated and no larger than 10 × 10 cm2.67 It occurs mainly on the face and upper extremities of otherwise healthy individuals. Profuse sweating, which is usually precipitated by heat, lasts 15–60 minutes. Mental or gustatory stimulation also triggers sweating. There is no accompanying sensory or motor neuropathy, flushing of the face, headaches, excessive salivation, lacrimation, vasodilation, or piloerection. The pathogenesis of circumscribed hyperhidrosis is unknown. Sweating may be partially controlled by local application of 20% aluminum chloride in ethanol, topical anticholinergic agents, systemic clonidine (which inhibits central sympathetic outflow), or by local injections of BTX.
The involved area in idiopathic unilateral circumscribed hyperhidrosis is usually sharply demarcated and no larger than 10 × 10 cm2.67 It occurs mainly on the face and upper extremities of otherwise healthy individuals. Profuse sweating, which is usually precipitated by heat, lasts 15–60 minutes. Mental or gustatory stimulation also triggers sweating. There is no accompanying sensory or motor neuropathy, flushing of the face, headaches, excessive salivation, lacrimation, vasodilation, or piloerection. The pathogenesis of circumscribed hyperhidrosis is unknown. Sweating may be partially controlled by local application of 20% aluminum chloride in ethanol, topical anticholinergic agents, systemic clonidine (which inhibits central sympathetic outflow), or by local injections of BTX.
![]() Older women, much more so than men, are occasionally troubled by a syndrome of daytime, paroxysmal hyperhidrosis primarily affecting the head, neck, and upper trunk. Hot flashes (head and neck heat and flushing) are not commonly associated, and hormonal replacement therapy is usually ineffective. Nevertheless, affected women are often postmenopausal and have previously experienced typical hot flashes. Sweating before menopause is normal, distinguishing the syndrome from craniofacial essential hyperhidrosis. Normal whole body thermoregulatory sweating rules out compensatory hyperhidrosis. Alterations in hypothalamic set point temperature range for sweating are suspect in many cases. Symptomatic treatment with clonidine, glycopyrrolate (0.5%–2.0% topical or 3–8 mg/day orally), or with other agents (scopolamine, phenobarbital, topiramate) may be effective. Whether this syndrome is simply an “aged” version of typical hot flashes or a late onset idiopathic primary focal hyperhidrosis is uncertain.
Older women, much more so than men, are occasionally troubled by a syndrome of daytime, paroxysmal hyperhidrosis primarily affecting the head, neck, and upper trunk. Hot flashes (head and neck heat and flushing) are not commonly associated, and hormonal replacement therapy is usually ineffective. Nevertheless, affected women are often postmenopausal and have previously experienced typical hot flashes. Sweating before menopause is normal, distinguishing the syndrome from craniofacial essential hyperhidrosis. Normal whole body thermoregulatory sweating rules out compensatory hyperhidrosis. Alterations in hypothalamic set point temperature range for sweating are suspect in many cases. Symptomatic treatment with clonidine, glycopyrrolate (0.5%–2.0% topical or 3–8 mg/day orally), or with other agents (scopolamine, phenobarbital, topiramate) may be effective. Whether this syndrome is simply an “aged” version of typical hot flashes or a late onset idiopathic primary focal hyperhidrosis is uncertain.
![]() Localized hyperhidrosis has been reported to occur in the skin over a blue rubber bleb nevus, in the perilesional skin of a glomus tumor (presumably due to increased local temperature and/or pain), and in POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) syndrome, Gopalan disease (burning feet syndrome), pachydermoperiostosis, and painful pretibial myxedema. Hyperhidrosis is also often associated with eccrine angiomatous hamartoma, tufted angioma, a vascular tumor, and with a nevoid proliferative condition that shows increased numbers of eccrine glands and dilated vascular channels in the deep dermis and subcutaneous tissue on histologic evaluation of lesional tissue.68 The hamartoma is most commonly localized to an extremity, and usually presents as a solitary, sometimes painful, blue–purple, slow-growing nodule at birth or in childhood, but may first be noted in adults.
Localized hyperhidrosis has been reported to occur in the skin over a blue rubber bleb nevus, in the perilesional skin of a glomus tumor (presumably due to increased local temperature and/or pain), and in POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) syndrome, Gopalan disease (burning feet syndrome), pachydermoperiostosis, and painful pretibial myxedema. Hyperhidrosis is also often associated with eccrine angiomatous hamartoma, tufted angioma, a vascular tumor, and with a nevoid proliferative condition that shows increased numbers of eccrine glands and dilated vascular channels in the deep dermis and subcutaneous tissue on histologic evaluation of lesional tissue.68 The hamartoma is most commonly localized to an extremity, and usually presents as a solitary, sometimes painful, blue–purple, slow-growing nodule at birth or in childhood, but may first be noted in adults.
Secondary Causes of Generalized Hyperhidrosis
![]() Episodic hypothermia [core temperature <35°C (95°F)] with hyperhidrosis (Shapiro syndrome) was originally described in association with agenesis of the corpus callosum,69 but has more recently been reported in association with human immunodeficiency virus. However, it may occur without an identifiable brain lesion or systemic illness, and can affect both children and adults. The hypothermia may be due to periodic dysfunction of the medial preoptic hypothalamic region with lowering of the “set-point” temperature, producing profuse sweating to lower core temperature. Treatment with anticonvulsants, oxybutynin, clonidine, and glycopyrrolate have been used with variable success.70–72
Episodic hypothermia [core temperature <35°C (95°F)] with hyperhidrosis (Shapiro syndrome) was originally described in association with agenesis of the corpus callosum,69 but has more recently been reported in association with human immunodeficiency virus. However, it may occur without an identifiable brain lesion or systemic illness, and can affect both children and adults. The hypothermia may be due to periodic dysfunction of the medial preoptic hypothalamic region with lowering of the “set-point” temperature, producing profuse sweating to lower core temperature. Treatment with anticonvulsants, oxybutynin, clonidine, and glycopyrrolate have been used with variable success.70–72
![]() Generalized hyperhidrosis without hypothermia has been reported in patients with episodic hypertension, tachycardia, flushing, and hypothalamic-pituitary dysfunction after brain injury, infarction, or tumor in the region of the hypothalamus. These “autonomic storms” or “diencephalic epileptic” events are not true seizures but likely reflect overactivity or disinhibition of the hypothalamic areas, which control the sympathoexcitatory stress reaction. Hyperhidrosis has also been reported with fatal familial insomnia (an autosomal dominant prion disease clinically characterized by inattention, sleep loss, dysautonomia, and motor signs). Involvement of the medial thalamic nuclei and connecting “limbic” system pathways may be causative.73 In Parkinson disease, hyperhidrosis is a frequent manifestation during dopamine drug “off” states.74
Generalized hyperhidrosis without hypothermia has been reported in patients with episodic hypertension, tachycardia, flushing, and hypothalamic-pituitary dysfunction after brain injury, infarction, or tumor in the region of the hypothalamus. These “autonomic storms” or “diencephalic epileptic” events are not true seizures but likely reflect overactivity or disinhibition of the hypothalamic areas, which control the sympathoexcitatory stress reaction. Hyperhidrosis has also been reported with fatal familial insomnia (an autosomal dominant prion disease clinically characterized by inattention, sleep loss, dysautonomia, and motor signs). Involvement of the medial thalamic nuclei and connecting “limbic” system pathways may be causative.73 In Parkinson disease, hyperhidrosis is a frequent manifestation during dopamine drug “off” states.74
Malaria, tuberculosis, brucellosis, and subacute bacterial endocarditis may present with fever and generalized hyperhidrosis due to exogenous bacterial pyrogens that stimulate phagocytic leukocytes to produce endogenous pyrogen [interleukin 1 (IL-1) and IL-6, tumor necrosis factor (TNF), and γ-interferon]. These cytokines act not only as circulating hormones, but also as intrinsic modulators in the brain. Signals that stimulate IL-1 production in the brain include humoral factors, such as circulating IL-6 and activation of peripheral C fibers and vagal afferents. These effects can raise “set-point” temperature (producing fever), while simultaneously activating antipyretic mechanisms (eventually producing drenching sweats).75
Increased sweating has been reported in diabetes mellitus, hypoglycemia, congestive heart failure, thyrotoxicosis, hyperpituitarism, dumping syndrome, carcinoid syndrome, and alcohol and drug withdrawal. Sweating is increased (especially in males) in acromegaly (in which the size of sweat gland acini and the density of innervation to the sweat glands is greater than in controls) and is decreased in growth hormone deficiency (GHD).
Some studies of GHD pre- and posttreatment, using histochemistry for acetylcholinesterase and immunohistochemistry for the neuropeptide vasoactive intestinal polypeptide and protein gene product 9.5, a general neural marker, have shown increased sweat gland acinar size and periacinar innervation and pilocarpine iontophoresis sweat rate after GH therapy. However, other studies have shown that even treated GHD subjects still sweat less than controls implying permanent eccrine gland hypofunction and androgen deficiency as a cofactor of reduced sweating.
Hodgkin disease is characterized by the triad of fever, sweating, and weight loss; night sweats may be the only symptom and 31 of 100 patients present with “B”-cell symptoms (fever, weight loss, sweating).76 The excessive production of IL-1 by activated macrophages is implicated as the cause of temperature instability.77 IL-1 is known to induce an abrupt increase in the synthesis of prostaglandin E2 in the preoptic anterior hypothalamic region, causing an elevation of the temperature “set point.” Excessive production of IL-6 by Hodgkin lymphoma cells78 is also implicated as cause of fever and subsequent night sweating. Advanced solid tumors may also cause sweating via immunologic mechanisms relating to TNF-α and effect of ILs on central thermoregulation.
The symptomatic triad of excessive and inappropriate paroxysmal sweating, tachycardia, and pounding headaches (associated with increased blood pressure) almost assures the diagnosis of pheochromocytoma as the cause of hyperhidrosis. Anti-α- and β-adrenergic therapy is a mainstay of this disorder, with a rare patient developing sweat gland necrosis during preoperative therapy.
Hyperhidrosis is frequently associated with serotonin (5-hydroxytryptamine) reuptake inhibitors, opioids, and sometimes with prostaglandin inhibitors (naproxen). The serotonin syndrome and the neuroleptic malignant syndrome include hyperthermia, labile blood pressure, hyperhidrosis, rigidity, agitation and confusion. The mechanisms may relate to 5-hydroxytryptamine (2A) and dopamine receptor antagonism. The hyperhidrosis that commonly occurs during acute and chronic administration of opioids is mainly due to stimulation of mast cell degranulation, resulting in the release of histamine.79 Excessive sweating can occur in as much as 45% of patients taking methadone.80 Hyperhidrosis is also a recognized side effect of transdermal fentanyl.81 Sweating in combination with hypertension, nausea, and mydriasis characterizes acute opioid and alcohol withdrawal. Despite their well-recognized anticholinergic effects, tricyclic antidepressants occasionally cause hyperhidrosis due to their sympathomimetic effect. The presumed mechanism is inhibited reuptake of norepinephrine, leading to stimulation of peripheral adrenergic receptors and a generalized diaphoretic response. Cholinergic agonists such as pilocarpine and bethanechol and reversible cholinesterase inhibitors such as pyridostigmine can increase sweating directly or indirectly via activation of M3 cholinergic receptors on sweat glands. See Table 84-2 for an abbreviated listing by drug class, example, and mechanism of hyperhidrosis and Table 84-3 for a complete listing of medications reported to cause hyperhidrosis.
Class | Examples | Probable Mechanism |
|---|---|---|
Anticholinesterases | Pyridostigmine | Cholinesterase inhibition |
Antiglaucoma agents | Physostigmine Pilocarpine | Cholinesterase inhibition for physostigmine; muscarinic cholinergic agonism for pilocarpine |
Bladder stimulants |