Diagnostic and Therapeutic Techniques
Amanda E. Zubek
Lacey L. Kruse
Jacqueline A. Guidry
Christopher G. Bunick
Kelly J. Stankiewicz
CYTOLOGIC SMEARS
I. BACKGROUND
Cytologic techniques in dermatology are useful in the diagnosis of bullous diseases, vesicular viral eruptions, and molluscum contagiosum. Other diseases that are also amenable to diagnosis by cytology include some genodermatoses such as Hailey-Hailey and Darier; drug hypersensitivity such as toxic epidermal necrolysis (TEN); infections such as staphylococcal scalded skin syndrome (SSSS) and leishmaniasis; and even tumors such as basal cell carcinoma (BCC), mastocytoma, and squamous cell carcinoma. However, these techniques require more experience in their interpretation. The cytologic smear technique allows for rapid confirmation of a suspected diagnosis while awaiting histopathologic processing and interpretation of biopsies.
II. TECHNIQUE
A. Select an Early Lesion that shows no signs of trauma or infection.
B. Separate or Remove the Blister Top with a scalpel or sharp scissors. Absorb excess fluid with a gauze pad.
C. Gently Remove the Blister Contents and scrape the floor and edges of the vesicle with a no. 10 or 15 scalpel blade or curette.
D. Make a Thin Smear on a clean glass slide. With solid lesions such as molluscum, squeeze the material between two slides.
E. Air-Dry. If the following reagents are available, fix tissue by dipping it four to five times in 95% ethanol or methanol or immerse the slide in these solutions for 1 to 2 minutes.
F. Stain with Wright, Giemsa (one-half dilution with tap water for 30 to 40 seconds), or hematoxylin and eosin stain. Ideally, 20- to 25-minute incubation is preferable for Wright stain.
G. Microscopic Appearance. Examine first with a low-power objective to gain an impression of cell size and depth of stain relationships, then examine with 45× or oil objective for the morphologic details.
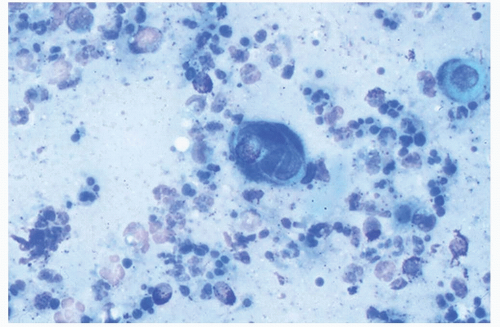
1. Herpetic Viral Infections. The Tzanck smear demonstrates multinucleated giant cells with nuclear molding, occasional nuclear inclusions, blurred chromatin (“ballooning degeneration”), and occasional atypicalappearing mononucleate cells (Fig. 49-1). These giant cells represent infected keratinocytes. Without direct immunofluorescent antibody staining, it is impossible to differentiate between herpes simplex virus and varicella-zoster virus. Between 60% and 70% of Tzanck smears show the characteristic changes of herpesvirus infection. Occasionally, intranuclear inclusion bodies may be identified.
2. Molluscum Contagiosum. Henderson-Patterson inclusion bodies (virus-transformed keratinocytes) appear as multiple, large, oval to round, smooth-bordered basophilic masses up to 25 µm in diameter.
3. In Some Bullous Eruptions, Acantholytic Rounded Keratinocytes Are Seen in the cytologic smear. When determining between SSSS and TEN, the presence of necrotic keratinocytes and inflammatory cells points toward a diagnosis of TEN.
4. In BCC, Tzanck Smear Shows Clustered Basaloid Cells, often with peripheral palisading.
FUNGAL SCRAPING AND CULTURE
I. BACKGROUND
Two techniques are available for diagnostic confirmation of a fungal infection: direct microscopy and fungal cultures. Immediate confirmation of the presence of a fungal infection may be accomplished easily by microscopic identification of organisms. Fungal culture will identify the causative organism specifically. This is therapeutically important because some nondermatophytic molds such as Scytalidium hyalinum as well as Candida species may mimic dermatophytes on potassium hydroxide (KOH) examination but are often resistant to conventional dermatophyte therapy.
II. TECHNIQUE
All scaling lesions from the scalp, angles of the mouth, axillae, groin, inframammary area, and feet, as well as blisters on the hands and feet, should be considered for both fungal scraping and fungal culture.
A. Scraping Examination
1. Skin
1. If lesions are soiled or macerated, clean the skin well with alcohol and let dry.
2. Scrape with a scalpel or edge of a microscope slide at the active border of a lesion and collect scales on a glass slide. With blistering eruptions, the fungus is in the roof of the vesicle, which can be either gently dissected off with sharp scissors or scalpel or reflected back and the underside scraped with a no. 15 scalpel blade.
3. When obtaining culture specimens from anxious or uncooperative patients, vigorous rubbing of the lesion with a moistened sterile cotton swab provides an effective atraumatic alternative to actual scraping.1
4. Small, thin fragments of tissue may be examined directly. Large pieces should be minced with a scalpel blade. Thick pieces should be discarded.
5. Gather scrapings together in the center of the slide.
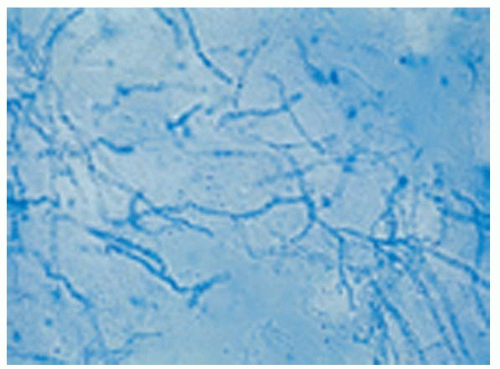
6. Mix with one to two drops of 10% to 20% KOH. Add a coverslip and heat gently, but not to boiling, for 15 seconds. Note that two types of KOH are available: one in water and the other in dimethyl sulfoxide (DMSO). The KOH in DMSO does not require heating. An alternative preparation is covering the scrapings with either chlorazol black E stain or Swartz-Lamkins stain, which stain fungal hyphae, providing a contrast between fungi and background cells (Fig. 49-2).
7. Let KOH slides cool for 10 minutes (during which time the tissue is hydrolyzed and rendered clear) and then press the coverslip gently to flatten the tissue and push out air bubbles.
8. Examine under a scanning lens or high-dry magnification. It is very important to avoid getting KOH on the microscope objective, as it will etch the lens. The diaphragm should be closed down and the condenser lowered as far as possible. The ease of identification of hyphae varies inversely with the intensity of light passing through the slide.
9. In KOH preparations, hyphae and spores will appear as refractile tubes and oval bodies against the background of cells and debris. It is not possible to make a species identification from tissue scrapings.
Using Swartz-Lamkins stain, hyphae and spores appear blue against the unstained background of cells. This fungal stain consists of a dye, a surfactant to clear the tissues quickly, and less KOH (2%) content to avoid damaging microscope lenses. Chlorazol black preparations will stain hyphae green against a cellular background. Because the hyphae stain selectively and are seen more easily with these stains, slides may be scanned more quickly at a lower power.2
2. Hair and Nails
1. Examine the scalp with a Wood’s lamp. If individual lesions fluoresce, pull out 10 to 15 hairs for examination. Otherwise, examine scales and 10 to 15 randomly plucked hairs from the involved site. Fungus invades the hair in two ways: ectothrix involvement, where the hair shaft is surrounded by tiny spores, and endothrix infection, where the spores are found inside the hair shaft. Ectothrix fungi include Microsporum species as well as Trichophyton mentagrophytes and Trichophyton verrucosum. Endothrix infections are seen with Trichophyton tonsurans, Trichophyton violaceum, and Trichophyton schoenleinii. Altered, dystrophic, hypertrophic, or pigmented nails should be snipped off and minced on a slide.3 Subungual debris is less suitable for direct examination, though it is preferable for fungal culture.
2. Heat the specimen on a slide or in a test tube with 10% to 40% KOH and let cool for 15 to 30 minutes. Tissue may then be stained or examined directly.
B. Culture. In addition to direct examination of scales, scrapings from a suspicious lesion should be cultured at room temperature on Sabouraud glucose agar, Sabouraud agar with chloramphenicol and cycloheximide (Mycobiotic, Mycosel), or dermatophyte test medium (DTM).
Sabouraud agar with chloramphenicol and cycloheximide is more selective for dermatophytes because the chloramphenicol and cycloheximide inhibit bacteria and other contaminants. DTM contains cycloheximide, gentamicin, and chlortetracycline, preventing the growth of bacteria and saprophytic fungi. DTM contains phenol red, which turns the agar from yellow to bright red when its pH becomes alkaline from dermatophyte growth. Contaminant growth does not alter the pH of the medium. Using DTM medium, the specimen should be inoculated onto the media and the cap loosely placed, thereby providing the fungi with the air they require for growth. If no color change takes place within 2 weeks, the culture may be discarded. If the color does change, it may be presumed that a pathogenic dermatophyte or yeast is present. Microscopic examination of the culture (culture mount) should then identify the exact species.
WOOD’S LAMP EXAMINATION
I. BACKGROUND
Invented in 1903 by Robert W. Wood, Wood’s lamp uses a light source with a glass filter containing barium silicate and 9% nickel oxide to emit bands of light between 320 and 400 nm (peak 365 nm). The light source may be from a fluorescent tube, a mercury vapor lamp, a light-emitting diode, or an incandescent light. Tissue fluorescence occurs when Wood’s light is absorbed and visible light is emitted; however, this is minimal in normal skin due to the presence of amino acids, elastin, and melanin. Fluorescent bulbs
(black lights) emitting a similar, although slightly broader, spectrum light are also available.
(black lights) emitting a similar, although slightly broader, spectrum light are also available.
II. TECHNIQUE
Initially used for the detection of fungal infection, Wood’s lamp examination may also be useful in clinical settings where bacterial infection, pigmentary disorders, porphyria, tetracycline use, or exposure to fluorescent materials is suspected (Tables 49-1 and 49-2).
A. Detection of Scalp Tinea. Hairs infected with Microsporum audouinii, Microsporum canis, or Microsporum distortum fluoresce bright blue-green under Wood’s lamp. Fluorescent hairs may then be selected for microscopic examination and culture. Unfortunately, the emergence of T. tonsurans, a nonfluorescent dermatophyte, as the most common cause of tinea capitis limits the usefulness of screening with Wood’s lamp examination.
B. Detection of Other Fungal Infections. While often clinically imperceptible, tinea versicolor fluoresces golden yellow. Wood’s light examination will allow the accompanying pigmentary changes to be seen more vividly.
C. Detection of Bacterial Infections
1. Erythrasma, an intertriginous infection caused by Corynebacterium minutissimum, fluoresces brilliant coral red or pink-orange. The fluorescent substance is a water-soluble porphyrin that may be washed off the skin causing a falsely negative Wood’s lamp examination.
2. Pseudomonas aeruginosa infections produce a yellow-green fluorescence due to pyocyanin. Its fluorescence is detectable before obvious purulence appears. Therefore, Wood’s lamp examination is a useful screening tool for infections in burn patients.
TABLE 49-1 Proper Technique of Wood’s Lamp | |
|---|---|
|
TABLE 49-2 Diagnostic Uses of Wood’s Lamp | |
|---|---|
|
D. Delineation of Pigmentary Disorders. Epidermal melanin prevents transmission of Wood’s light past the epidermis in normal skin. Therefore, variations in epidermal pigmentation (freckles, melasma, and vitiligo) are more apparent under Wood’s lamp while variations in dermal pigmentation (Mongolian spot, some instances of postinflammatory hyperpigmentation) are less apparent or unchanged as compared with ambient light.
If transmitted to the dermis, Wood’s light will cause dermal collagen to fluoresce white to blue. Thus, Wood’s light can distinguish hypopigmented from amelanotic areas. Amelanotic areas will have true white to blue fluorescence since there is no epidermal melanin to interfere with Wood’s light transmission. Wood’s lamp can be used to examine patients with vitiligo, albinism, leprosy, and other disorders of hypopigmentation and to screen newborns for the small, ash-leaf-shaped, white macules indicative of tuberous sclerosis.
E. Detection of Porphyrins. Acidified urine, feces, and, rarely, blister fluid from patients with porphyria cutanea tarda will fluoresce brilliant pinkorange.
F. Drug Detection. Patients who ingested tetracycline during childhood may have teeth that fluoresce yellow. Topical tetracycline therapy will cause treated sites to fluoresce yellow, while oral tetracycline therapy may cause pink fluorescence of the nail bed lunulae.
G. Miscellaneous. Fluorescent ingredients or markers in cosmetics, medications, or industrial compounds may be detected with Wood’s lamp examination.
PATCH TESTING