6 Diagnosis and treatment of painful neuroma and of nerve compression in the lower extremity
Synopsis
Introduction
With the exception of harvesting the sural nerve from the leg to use as a graft for various upper extremity nerve reconstructions, or for cross-facial nerve grafting, the peripheral nerve component of the lower extremity has been largely ignored. To be sure, orthopedic or podiatric foot and ankle surgery textbooks have an obligatory chapter on the tarsal tunnel syndrome and Morton’s neuroma, but relatively little else. Mackinnon and Dellon, in 1989, attempted to apply those principles established for upper extremity peripheral nerve surgery to the lower extremity,1 addressing, for example, the problems related to the tibial nerve at the level of the ankle, and the problems related to the peroneal nerve at the knee, leg, and foot level, as well as the problems with the sural nerve and the interdigital nerve.
Historical perspective
The history of peripheral nerve surgery related to the painful neuroma and chronic nerve compression is well documented.1–5 It should be noted that the terms “causalgia” and “reflex sympathetic dystrophy” are replaced by “complex regional pain syndrome I” and “complex regional pain syndrome II,” respectively, according to modern usage in the pain management community.
Basic science/disease process
The painful neuroma
A peripheral nerve is covered with a myelin sheath produced by a Schwann cell.
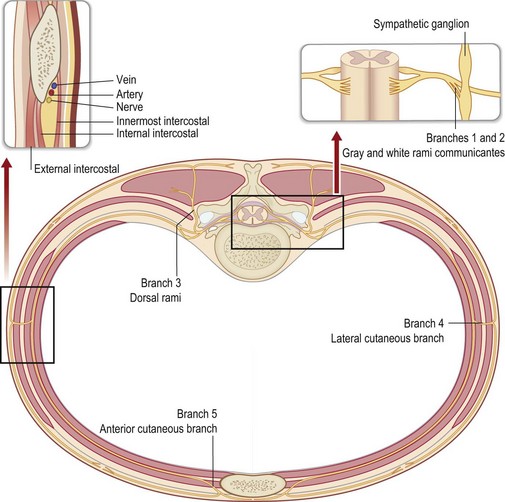
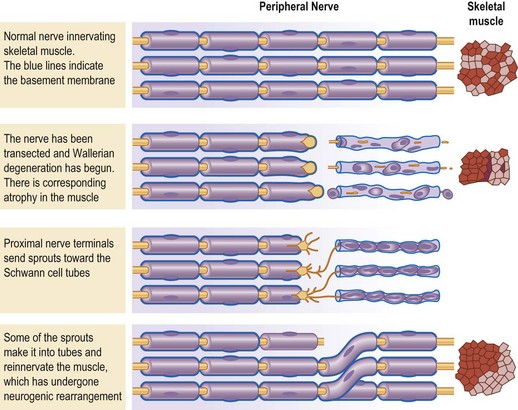
The cell body for a sensory neuron of the peripheral nervous system lies in a dorsal root ganglion and from the exit of this nerve from the vertebral foramen it is covered with Schwann cells (Fig. 6.1). This is in contrast to the true cranial nerves with sensory function, like the optic and olfactory nerves, which are extensions of the central nervous system, and do not regenerate successfully to the distal target organ.6 When a peripheral nerve is injured, for example by a complete transection, the distal portion of the nerve degenerates (Wallerian degeneration).7 Since the Schwann cell is a single cell living along the length of the degenerating axon of the peripheral nerve, the Schwann cell remains alive. Ultimately, all that will remain of the nerve is the basement membrane that was elaborated by the Schwann cell. When the interaction between the axon and the Schwann cell is lost, the Schwann cell up-regulates and produces nerve growth factor.8 The proximal end of the peripheral nerve will produce axon sprouts within 24 hours of the nerve transection, which will begin to regenerate distally. The sprouts are lured by the nerve growth factor, and will track preferentially along the basement membrane, for whose negatively charged laminin and type IV collagen the sprouts have an affinity (Fig. 6.2).9–11 The regeneration will proceed along the distal pathway towards the appropriate target end-organ.12
If this process is thwarted at the site of neural regeneration, a neuroma will form. A neuroma is a benign tumor created by axon sprouts becoming encased in collagen. A neuroma will become painful if it is located in an area that subjects these sprouts to stimulation. Such sites include locations that are superficial, next to joints, adherent to moving tendons, and subjacent to weight-bearing surfaces. The common denominator for all of these is tension. The tension causes the fibroblasts in the region of the axon sprouts to produce the collagen that results in the growth of the neuroma and the adherence of the neuroma to surrounding structures.13
Within this evolving mass of axon sprouts and fibroblasts, abnormal communications or synapse-like structures may form between nerves that normally do not communicate with each other. These are the source of ephaptic conduction, which has been shown to be the origin of pain signals from the small myelinated group A-delta and unmyelinated group C fibers. Spontaneous origin of signals from these pain and temperature fibers have been recorded by single-unit neurophysiologic techniques from neuromas of the radial sensory nerve in the baboon.14 For example, that study found that 10% of more than 200 fibers that were studied had spontaneous activity in a 2-month neuroma, and this percentage increased to 18% in a 7-month neuroma, which was twice the activity found in the same-diameter intact (control) nerves. That study also demonstrated that the neuroma was mechanosensitive; just touching it generated signals, which did not occur from the control nerves. These findings provide the basis for the author’s current treatment strategy that involves resecting the end-bulb neuroma, instead of the traditional approach that involved just moving the “mature end-bulb neuroma” to a new place that was “quiet.”15–17
Chronic nerve compression
The pathophysiology of chronic nerve compression implies that the evaluation of nerve function must be based upon the knowledge that there will be a progressive loss of peripheral nerve function. Therefore, the diagnosis and treatment of chronic nerve compression are based upon an understanding of this pathophysiology. The structure and function of the peripheral nerve, and how these are altered with increasing degrees of pressure and with increasing lengths of time, have been investigated in rat, rabbit, and monkey animal models,18–28 and many of these models have used the sciatic nerve. Therefore, the disease process is the same for the better-known upper extremity nerve compressions as it is for the lesser-known lower extremity peripheral nerve compressions.
A review of this literature indicates that if there is a constriction of greater than 20% placed upon the diameter of the peripheral nerve, then acute axonal degeneration will occur. If there is a pressure of greater than 20 mmHg applied to the peripheral nerve, there will be a decrease in blood flow. In the acute situation, these conditions produce pain. If this degree of compression comes on over a gradual period of time, and over a length of the peripheral nerve that is several times the diameter of the nerve, then the conditions produce chronic nerve compression. After about 2 months of compression by a silicone tube that does not constrict the diameter of the nerve, the first changes in the pathophysiology occur. These are a weakening of the tight junctions of the endothelial cells in the perineurium, and this results in serum entering the endoneurial space. This creates endoneurial edema. When this occurs in the relatively tight confines of the perineurium, there is an increase in pressure that will decrease blood flow. The conscious perception of this is paresthesia. At this point in time, the only clinical findings will be related to the cutaneous sensory threshold for touch, which will first become elevated for static two-point discrimination, or will manifest itself as a change in perception of vibratory stimuli applied with a tuning fork or a vibrometer.29,30 These pathophysiologic concepts provide a basis for staging chronic nerve compression (Tables 6.1–6.4).
Table 6.1 Numerical grading scale for any peripheral nerve32
| Grade | Description |
|---|---|
| 0 | Normal |
| 1 | Intermittent sensory symptoms |
| 2 | Increased sensorimotor threshold |
| 3 | Increased sensorimotor threshold |
| 4 | Increased sensorimotor threshold |
| 5 | Persistent sensory symptoms |
| 6 | Sensorimotor degeneration |
| 7 | Sensorimotor degeneration |
| 8 | Sensorimotor degeneration |
| 9 | Anesthesia |
| 10 | Muscle atrophy, severe |
Table 6.2 Numerical grading scale for the median nerve at the wrist level32
| Numerical score | Description of impairment | |
|---|---|---|
| Sensory | Motor | |
| 0 | 0 | None |
| 1 | Paresthesia, intermittent | |
| 2 | Abnormal pressure threshold (Pressure-Specifice Sensory Device) | |
| <45 years old: ≤3 mm, at 1.0–20 g/mm2 | ||
| ≥45 years old: ≤4 mm, at 2.2–20 g/mm2 | ||
| 3 | Weakness, thenar muscles | |
| 4 | Abnormal pressure threshold (Pressure-Specifice Sensory Device) | |
| <45 years old: ≤3 mm, at >20 g/mm2 | ||
| ≥45 years old: ≤4 mm, at 20 g/mm2 | ||
| 5 | Paresthesias, persistent | |
| 6 | Abnormal innervation density (Pressure-Specifice Sensory Device) | |
| <45 years old: ≥4 mm < 8 mm, at any g/mm2 | ||
| ≥45 years old: ≥5 mm < 9 mm, at any g/mm2 | ||
| 7 | Muscle wasting (1–2/4) | |
| 8 | Abnormal innervation density (Pressure-Specifice Sensory Device) | |
| <45 years old: ≥8 mm, at any g/mm2 | ||
| >45 years old: ≥9 mm, at any g/mm2 | ||
| 9 | Anesthesia | |
| 10 | Muscle wasting (3–4/4) | |
Table 6.3 Numerical grading scale for the ulnar nerve at the elbow level32
| Numerical score | Description of impairment | |
|---|---|---|
| Sensory | Motor | |
| 0 | 0 | None |
| 1 | Paresthesia, intermittent | |
| 2 | Weakness: pinch/grip (lb) | |
| Female: 10–14/26–39 | ||
| Male: 13–19/31–59 | ||
| 3 | Abnormal pressure threshold (Pressure-Specified Sensory Device) | |
| <45 years old: ≤3 mm, at 1.0–20.0 g/mm2 | ||
| ≥45 years old: ≤4 mm, at 1.9–20.0 g/mm2 | ||
| 4 | Weakness: Pinch/grip (lb) | |
| Female: 6–9/15–25 | ||
| Male: 6–12/15–30 | ||
| 5 | Paresthesia, persistent | |
| 6 | Abnormal innervation density (Pressure-Specified Sensory Device) | |
| <45 years old: ≥4 mm <8 mm, at any g/mm2 | ||
| ≥45 years old: ≥9 mm, at any g/mm2 | ||
| 9 | Anesthesia | |
| 10 | Muscle wasting (3–4/4) | |
Table 6.4 Numerical grading scale for the tibial nerve at ankle level
| Numerical score | Description of impairment | |
|---|---|---|
| Sensory | Motor | |
| 0 | 0 | None |
| 1 | Paresthesia, intermittent | |
| 2 | Abnormal pressure threshold (Pressure-Specified Sensory Device) | |
| <45 years old: ≤5.6 mm, at 1.0–20 g/mm2 | ||
| ≥45 years old: ≤7.8 mm, at 2.2–20 g/mm2 | ||
| 3 | Weakness, abductor hallucis | |
| 4 | Abnormal pressure threshold (Pressure-Specified Sensory Device) | |
| <45 years old: ≤5.6 mm, at 20 g/mm2 | ||
| ≥45 years old: ≤7.8 mm, at 20 g/mm2 | ||
| 5 | Paresthesias, persistent | |
| 6 | Abnormal innervation density (Pressure-Specified Sensory Device) | |
| <45 years old: ≥7 mm <10 mm, at any g/mm2 | ||
| ≥45 years old: ≥9 mm < 12 mm, at any g/mm2 | ||
| 7 | Intrinsic muscle wasting, clawing (1–2/4) | |
| 8 | Abnormal innervation density (Pressure-Specified Sensory Device) | |
| <45 years old: ≥11 mm, at any given g/mm2 | ||
| ≥45 years old: ≥15 mm, at any g/mm2 | ||
| 9 | Anesthesia | |
| 10 | Intrinsic muscle wasting, clawing (3–4/4) | |
After about 6 months of this degree of compression, the first histologic changes in myelin can be observed. This consists of thinning of the myelin. With progressive compression, there will be increasing loss of myelin, so that under slides in which a myelin stain has been used, it appears as if there has been a loss of the large myelinated, touch, fibers. They will not actually have begun to degenerate yet, as demonstrated by electron microscopy, in which the unmyelinated large fibers can be demonstrated still to be present. At this stage, the patient will have weakness in the motor system related to the muscles supplied by that nerve, and increasing numbness in the territory (fingers) related to the sensory supply of that nerve. Pinch and grip strength can be measured directly. There will be no atrophy, because without true loss of the motor axons, there will be no decrease in bulk of the muscles. At this stage of compression, the cutaneous pressure thresholds will be elevated increasingly for the quickly adapting fibers, measured either with vibrometry or with moving two-point discrimination with the Pressure-Specified Sensory Device (PSSD),31 and for the slowly adapting fibers, measured with static two-point discrimination with the PSSD. Although no longitudinal study using the Semmes–Weinstein nylon monofilaments has been published in patients with chronic nerve compression,33 the analogous measurement, made with the PSSD, which is one-point static touch, is still normal at this stage of compression.12 Two-point discrimination, measured in millimeters, is still normal, because no nerve fibers have died. At this stage in chronic nerve compression, with myelin thinning, traditional electrodiagnostic testing may begin to detect increases in the distal sensory latency. Amplitudes will still be normal because no nerve fibers have died yet. In general, quantitative neurosensory testing is more sensitive than traditional electrodiagnostic testing (nerve conduction study/electromyogram), so it is more likely to detect this stage of nerve compression. Neurosensory testing is less expensive than nerve conduction study/electromyogram, and will not cause the patient any pain.34,35
If either the length of time is increased for this degree of compression, or if the degree of compression increases, whether due to work, metabolic or rheumatologic disease, then neural degeneration occurs. As suggested in the preceding paragraph, this will be detected in the motor system by muscle wasting and in the sensory system by abnormal distance at which one from two points can be distinguished. Examples of these changes are given in Table 6.2 for median nerve compression at the wrist, Table 6.3 for ulnar nerve compression at the elbow, and Table 6.4 for tibial nerve compression in the tarsal tunnel.27,29 Using the PSSD, this change occurs first for static two-point discrimination and then for moving two-point discrimination. This was demonstrated in a retrospective analysis of patients with carpal and cubital tunnel syndrome. The one-point static touch measurement will become abnormal some time after the change in two-point discrimination distance, and is therefore a relatively insensitive tool for detecting the onset of this stage. Vibrometry and tuning fork use can identify this stage, but does not permit the therapist to identify correctly which nerve is being compressed without additional testing. It must be remembered that the vibratory stimulus travels as a wave, whereas the pressure stimulus remains localized to the applied test site.23,24,31 For example, if the involved nerve is the tibial nerve, and the hallux pulp is tested, there may still be relatively preserved sensation mediated by the peroneal nerve. Testing of both the dorsum of the foot and hallux pulp and medial heel may permit distinction of which nerve is involved.
Diagnosis/patient presentation
The painful neuroma
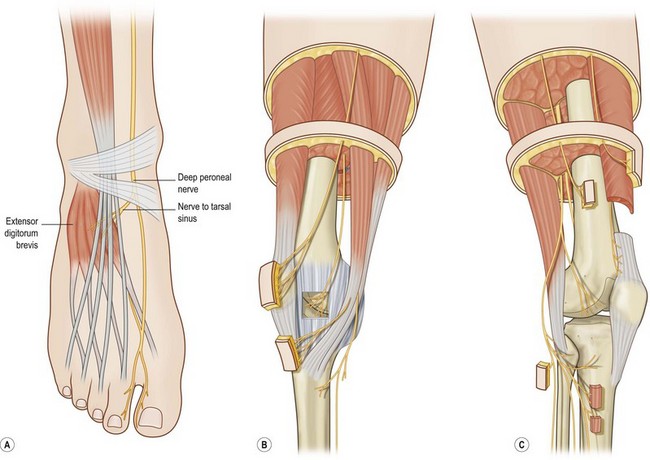
The patient with a painful neuroma will complain of an area of skin or a joint that causes pain either at rest, or, more commonly, when the part is touched or moved. When an area of pain relates to the territory of a peripheral nerve, and the patient has had an injury or an operation in that area, then consideration must be given to the presence of a painful neuroma of that peripheral nerve. In earlier times, this would have been called “causalgia,” but today, if the pain persists for more than 6 months, it is termed “complex regional pain syndrome I.” If movement of the ankle or knee creates pain, then consideration must be given to the presence of a neuroma of the nerve that innervates that joint. Anatomy books do not show nerves innervating joints, and this knowledge required cadaver dissections, which have been published for the knee (Fig. 6.3 B and C)36 and for the ankle (Fig. 6.3A).37 Clinical approaches and results for the lower extremity were reviewed in 2009.5 Beginning with the knee, and then extending this approach to the ankle, anatomical dissections were performed to identify joint innervation, With this knowledge routes for the administration of local anesthetic were identified. Demonstrating that pain relief is possible by anesthetic blockade in patients who failed traditional musculoskeletal approaches led to the creation of surgical approaches to resect involved nerve(s).
Chronic nerve compression
The patient with chronic compression of a peripheral nerve will complain of numbness or paresthesias in the skin territory innervated by that nerve. The patient will complain of weakness in the muscles innervated by that nerve. If both the peroneal and tibial nerves have chronic compression at the same time, the patient will have numbness over the dorsal and plantar aspects of the foot and arising proximal to the ankle. This will give the same appearance as if the patient had a peripheral neuropathy, especially if the problem occurs in both legs. This type of situation occurs in patients who have sports injuries, or other injuries. As a contrast in etiology, but with the same clinical presentation, will be patients who do have a metabolic neuropathy, for example, those with diabetes, or chemotherapy-induced neuropathy, who have secondary chronic nerve compressions. These patients with present with a history of neuropathy, and frequently will not have been examined to identify the presence of a chronic nerve compression.38 If the nerve compression is diagnosed and treated in such patients, they will frequently experience sensory recovery despite the presence of diabetic neuropathy.
The pathognomonic feature of chronic nerve compression on physical examination is the presence of a positive Hoffman–Tinel sign. With an understanding of the pathophysiology of chronic nerve compression (see above), it becomes clear that in the earliest stage there will be intermittent symptoms and there will be no physical findings present. This is often the situation with musicians, such as the pianist or violinist who complains of numbness in the little finger, from playing with the elbow bent, but has no Hoffman–Tinel sign over the ulnar nerve at the cubital tunnel. In the lower extremity, an analogous situation occurs with the soccer player whose leg may seem to give out, who has received many a blunt trauma to the outside of the knee or repeated ankle inversion sprains, and who has compression of the common peroneal nerve at the fibular neck. With further time and a greater degree of compression, axons will demyelinate and there will be an increase in the threshold required to produce a sensory response; a higher degree of vibration with the tuning fork, or a greater pressure required to discriminate one from two points touching the big-toe pulp with the PSSD while two-point discrimination distance remains normal. In the motor system, this will correspond to weakness on manual muscle testing. At this point, the Hoffman–Tinel sign will become positive. With further time and degree of compression, axons will die, and there will be a decrease in two-point static touch discrimination (the distance before two points can be distinguished will increase), and muscle atrophy will occur. The Hoffman–Tinel sign will remain positive. With a very advanced degree of compression, the peripheral nerve seems to stop its attempt to remyelinate and regenerate, and the Hoffman–Tinel sign becomes negative. This variation in the presence of the Hoffman–Tinel sign over time is understandable in terms of progression of compression and changes within the peripheral nerve, and should not be interpreted as a failure of the Hoffman–Tinel sign to be a valuable clinical examination technique.39,40