Dark under-eye circles are a common cosmetic complaint among patients, spanning all age groups and skin types. We review the anatomic and physiologic features of dark circles and highlight the varied treatment options available, including lasers to target pigment and superficial vasculature, fillers to reverse volume loss, and resurfacing to improve skin laxity and wrinkling.
Key points
- •
Under-eye dark circles are an unsurprising source of aesthetic concern of patients owing to the fatigued and less youthful appearance that they can impart.
- •
The etiology of under-eye circles is multifactorial and includes periorbital volume loss and skin laxity, orbital fat prolapse, increased prominence and density of subcutaneous vasculature, and excessive pigmentation.
- •
The ease of use, minimal incidence of complications, and lack of downtime associated with hyaluronic acid fillers make these products nearly ideal for treating infraorbital volume loss.
- •
Long-pulsed lasers target lower eyelid vasculature; Q-switched lasers and fractionated resurfacing treat cutaneous pigmentation. Skin laxity can be improved with fractionated resurfacing and microfocused ultrasound.
- •
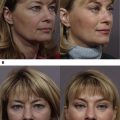
Standardized pretreatment and posttreatment digital photography is essential. Variations in lighting alone may mask or worsen lower eyelid appearance.
Introduction
Periorbital cutaneous and structural changes play a significant role in the perceived age of individuals of all ages and races. The relative darkening of lower eyelid skin, commonly referred to as under-eye (infraorbital) dark circles or periorbital hyperpigmentation, can impart a fatigued and less youthful appearance to the face. Dark circles are therefore an unsurprising source of significant aesthetic concern for a number of patients.
Introduction
Periorbital cutaneous and structural changes play a significant role in the perceived age of individuals of all ages and races. The relative darkening of lower eyelid skin, commonly referred to as under-eye (infraorbital) dark circles or periorbital hyperpigmentation, can impart a fatigued and less youthful appearance to the face. Dark circles are therefore an unsurprising source of significant aesthetic concern for a number of patients.
Anatomy
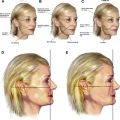
Any evaluation of lower eyelid dark circles must begin with an appropriate understanding of the underlying periorbital anatomy ( Fig. 1 ). The lower eyelid begins at the free palpebral margin and extends caudal to the inferior orbital rim, merging into the superior aspect of the cheek. It is bordered laterally by the lateral canthus and malar eminence and medially by the medial canthus and lateral nasal sidewall. The tear trough is an anatomic depression found in all age groups that extends obliquely from the medial canthus along the medial third of the lower eyelid. It is bound medially by the anterior lacrimal crest and inferiorly by the inferior orbital rim, lying within the limits of the orbicularis oculi muscle and corresponding to the anatomic location of the lacrimal sac. The tear trough forms the superior anatomic aspect of the nasojugal groove, which extends below the orbital rim.
Etiology
The formation of dark circles is often multifactorial, with a number of factors reported to play a role ( Table 1 ). A retrospective evaluation of periorbital hyperpigmentation in Southeast Asian patients revealed a predominantly vascular etiology, followed by constitutional (ie, periorbital melanosis), postinflammatory hyperpigmentation (PIH), and shadowing types.
| Type | Mechanism | Treatment Option |
|---|---|---|
| Hollowing/shadowing | Age-related infraorbital skin laxity and volume loss SOOF pseudoherniation Orbicularis oculi muscle hypertrophy | Hyaluronic acid filler Fractional ablative CO 2 laser resurfacing |
| Excessive pigmentation | Periorbital melanosis (“constitutional type”, may be an extension of pigmentary demarcation lines) Postinflammatory hyperpigmentation (allergic contact dermatitis, atopic dermatitis) Melasma Oculodermal melanocytoses (bilateral nevus of Ota-like macules) Rare: Acanthosis nigricans, fixed drug eruptions, and erythema dyschromium perstans | IPL Q-switched laser Nonablative fractionated resurfacing |
| Prominent vasculature | Thin, translucent skin Excess subcutaneous vascularity Venous stasis | Long-pulsed laser IPL Hyaluronic acid filler Fractional ablative CO 2 laser resurfacing |
| Exogenous | Penicillamine-induced periorbital pigmentation Bimatoprost-induced periorbital hollowing and hyperpigmentation | Hyaluronic acid filler Fractional ablative CO 2 laser resurfacing IPL Q-switched laser |
Shadowing Effect
Infraorbital skin laxity and volume loss with subcutaneous fat atrophy result from a combination of advancing age and chronic photodamage. These factors, along with hypertrophy of orbicularis oculi muscles, pseudoherniation of suborbicularis oculi fibroadipose tissue, and/or volume loss of the malar cheek, create a shadowing effect on the tear trough. This shadowing is lighting dependent, often masked with the use of direct flash photography.
Excessive Pigmentation
Excessive pigmentation of the lower eyelids owing to a number of underlying causes can also lead to under-eye circle formation. Melasma is an acquired facial hypermelanosis common in Southeast Asian and Hispanic populations with Fitzpatrick skin types III-IV that may predominate in the infraorbital areas. UV light exposure, pregnancy, exogenous hormones (including oral contraceptives), and genetic predisposition all likely play a role. Nevi of Ota in Asian populations are either congenital or develop during childhood and are thereby easily differentiated.
Orbital Lipodystrophy from Prostaglandin F2a
Periorbital changes have also been reported with ophthalmic and topical use of the prostaglandin F2a analogs, including bimatoprost 0.03% (Lumigan or Latisse; Allergan, Inc, Irvine, CA), travoprost, or latanoprost. An acquired orbital lipodysotrophy characterized by hollowing of lid sulci may rarely develop from local adipocyte atrophy owing to the potent anti-adipogenic effects of prostaglandin F2a. Improvement is typically noted after cessation of therapy or change to an alternative prostaglandin analog. Ophthalmic use of prostaglandin F2a analogs can also lead to reversible periocular hyperpigmentation owing to increased melanogenesis. The risk of pigmentation seems to be dramatically lower with topical bimatoprost for eyelash hypotrichosis because of minimal cutaneous contact via brush application.
Subcutaneous Fat and Veins
The minimal infraorbital subcutaneous fat, superficial location of the orbicularis oculi muscle, and thin, translucent skin of the lower eyelid can impart a violaceous appearance to the entire area as a result of prominent underlying intramuscular vasculature. Excess subcutaneous telangiectatic and reticular veins may also play a role. Greater dermal vessel congestion and stasis-related extravasation during episodes of physical and mental stress, including menstrual periods and pregnancy, may also worsen dark under-eye circles.
Evaluation
A thorough history and clinical assessment of the lower eyelids and cheeks is necessary to determine the underlying cause of a patient’s dark circles, choose the most appropriate course of treatment, and avoid complications. A history of ocular procedures, trauma, or allergies and the presence of autoimmune and neuromuscular disorders should first be elicited. Genetic or acquired diseases that may predispose patients to bleeding and infections must also be ruled out. Depending on individual underlying factors, a baseline comprehensive ocular examination may be warranted.
The cutaneous lower eyelids should be evaluated systematically to maximize consultation time and prevent oversights. Both epidermal and dermal features (textural irregularities, dyspigmentation, photodamage, atrophy, or vessel prominence) and subcutaneous findings (atrophy, hollowing, or prolapse) ought to be assessed. The presence of bilateral asymmetry should be noted. A detailed history and review of prior photographs can help to distinguish normal anatomic variation from age-related changes. Imaging with the VISIA system (Canfield Scientific, Inc, Fairfield, NJ) can highlight blood vessels and pigmentation with UV light and cross-polarized flash photography ( Fig. 2 ).
Evaluating and documenting the presence of a prominent tear trough deformity is essential. The medial and central aspects of the tear trough may be accentuated with an upward gaze, whereas the lateral border may be accentuated with an upward outward gaze contralaterally. Moreover, the importance of standardized, high-quality pretreatment and posttreatment digital photography with appropriate lighting cannot be overstated, given that different lighting conditions may mask or accentuate tear troughs and other aspects of the lower eyelids ( Fig. 3 ). Manual stretching of lower eyelid skin can help to differentiate between true pigmentation and shadowing effect. Although the former retains its appearance with stretching, the latter improves or resolves entirely. An increase in violaceous discoloration on manual stretching of the lower eyelids, on the other hand, is consistent with a translucent skin and/or hypervascular etiology.
Eyelid skin laxity and tone should also be properly assessed before any procedure using distraction or snap tests, respectively. Extensive lower eyelid laxity and/or orbital fat herniation on evaluation precludes the benefits of noninvasive treatment options, warranting operative intervention.
Procedures
Preoperative
A history of keloids, conditions that may impair wound healing, recent oral retinoid use, pregnancy, breastfeeding, photosensitivity, and/or abnormalities localized to the treatment area (active infections, malignant lesions, scarring, or burns) should be ruled out before undertaking any procedure. Any nonessential medications or supplements that may predispose patients to bleeding should be stopped if possible 2 weeks before any injectable treatment. Given the area, prophylactic antiviral therapy for herpes simplex virus is not routinely performed.
All patients should have photographs and written informed consent obtained upon arrival. The treatment area is then washed with a neutral cleanser to remove any makeup or other impurities. Topical anesthesia is generally unnecessary given the limited treatment area. Moreover, tetracaine-based topical anesthetics may cause transient erythema and should be avoided before intense pulsed light (IPL) or vascular lasers.
Any laser or IPL treatment of lower eyelid skin within the borders of the bony orbital rim requires intraocular metal eye shields. Otherwise, disposable adhesive and stainless steel or polymer-based ocular shields of an appropriate, wavelength-specific optical density are used for IPL and laser treatments, respectively. IPL glasses or laser eyewear with an optical density of 5+ are also mandatory for device operators and ancillary staff.
Intense Pulsed Light
Lumenis IPLs (M22 or Lumenis One, Lumenis Ltd, Yokneam, Israel) emit 515- to 1200-nm wavelengths via interchangeable cutoff filters ranging from 560 to 755 nm. An integrated chilled sapphire crystal tip, a thin layer of cold gel, and periprocedural cold air cooling (Cryo 5, Cynosure, Westford, MA) combine to guarantee proper epidermal protection and minimal patient discomfort during treatment. The cold, water-based gel also enhances optical coupling between the crystal and treatment area, decreasing the index of refraction of light and leading to deeper energy delivery. Treatment parameters with a 35- × 15-mm crystal, individualized for each patient based on skin type, are described in Table 2 . Darker skin types should be treated with greater caution using higher cutoff filter, lower fluences, and/or double to triple pulsing with longer interpulse delays to help spare epidermal melanin.
| Fitzpatrick Skin Types | Settings Used | ||
|---|---|---|---|
| Cutoff Filter (nm) | Delay (ms) | Initial Fluences (J/cm 2 ) | |
| I-II | 560 | 10–15 | 15–19 (increased by 10%–20% with subsequent treatments, based on clinical response) |
| III-IV | 560 or 590 | 20–50 | 14–16 |
| V-VI | 695 or 755 | Triple pulsing | 14–16 |
For lower eyelid hyperpigmentation, 3.0-ms double pulsing is performed, with mild darkening of pigmented areas noted almost immediately posttreatment. Sequential 4.0-ms pulses are used for telangiectasias or diffuse erythema; transient vessel spasm is the treatment endpoint for discrete vascular lesions. Large-caliber telangiectasias or reticular veins (≤1 mm) can be safely double pulsed with higher fluences (19–26 J/cm 2 ) and longer pulse durations (4–12 ms) using a smaller 15- × 8-mm crystal. Minimal pressure should be applied against the skin with the hand piece to avoid compression of target vessels. A typical patient requires 1 to 3 sessions to achieve significant improvement, with subsequent semiannual maintenance treatments.
Twelve subjects treated with 2 to 4 sessions with an older version IPL (Quantum, Lumenis Ltd) with 2.6/4.0 ms double-pulsing (20 ms delay) and fluences of 36 to 37 J/cm 2 demonstrated significant lightening ( P = .024) of lower eyelid idiopathic hyperpigmentation, as rated by 7 independent observers. Pigment recurrence was not seen at the 1-year follow-up. All subjects experienced posttreatment PIH (mean, 6.7 months). A prior study by the same group found greater improvement in under-eye hyperpigmentation with fewer adverse events using IPL compared with Q-switched ruby laser (QSRL).
Other IPL systems may also be used, but treatment should proceed with more conservative settings because they have different pulse characteristics (eg, wavelength range, pulse duration, and energy distribution).
Q-Switched Lasers
Cutaneous melanin has a broad, polychromatic absorption spectrum that peaks in the UV range and declines steadily as a function of increasing wavelength ( Fig. 4 ). Although significantly attenuated past 755 nm, energy absorption is still likely with wavelengths up to 1064 nm, allowing for treatment of deeper pigment and darker Fitzpatrick skin types. Given that melanosomes have thermal relaxation times of less than 1 μs, ultra-short pulse durations are required to selectively confine photothermal and photoacoustic effects to these structures. Multiple Q-switched lasers with nanosecond (and recently picosecond) pulse durations and wavelengths within the absorption range of melanin are currently available. The typical clinical endpoint of these treatments is immediate lesion whitening without pinpoint bleeding. Lower energy settings should be used initially to minimize the occurrence of PIH.
Q-switched ruby lasers
The 694-nm wavelength of QSRLs is moderately absorbed by melanin, yet poorly absorbed by competing chromophores such as hemoglobin. Rapid delivery of high-intensity energy at this wavelength disrupts melanosomes within keratinocytes, melanocytes, and melanophages, making them ideal for pigmented epidermal and superficial dermal lesions in Fitzpatrick skin types I-II.
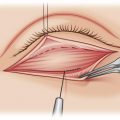
QRSL treatment is performed with 2 to 4 J/cm 2 using a 5-mm spot size (or varied accordingly) at 1.5 Hz ( Fig. 5 ). The clinical endpoint with this device is immediate lesion whitening that resolves over 20 minutes, followed by erythema and edema. Lowe and colleagues showed greater than 50% improvement in infraorbital hyperpigmentation in 23.5% and 88.9% of 17 subjects after 1 and 2 sessions, respectively. Another study of QSRL (6 to 7 J/cm 2 ) for under-eye dermal melanocytoses showed greater than 40% improvement in 4 subjects after 1 to 5 sessions. Combining QSRL with topical hydroquinone and tretinoin before and after treatment has also led to significant improvement in this location.
Q-switched alexandrite lasers
The more deeply penetrating 755-nm wavelength of the Q-switched alexandrite laser (QSAL) has a lower absorption coefficient for melanin and is emitted over a longer pulse duration (50–70 ns) than that of QSRL, which may serve to decrease adverse events (eg, PIH) in dark-skinned patients as a result of gentler melanosomal heating. QSAL treatments of Fitzpatrick skin types of IV or lower are typically performed with 3- to 5-mm spot sizes and 4 to 8 J/cm 2 . Lower fluences may lead to equal efficacy with decreased PIH.
A novel QSAL (PicoSure, Cynosure, Inc) with energy delivered in picoseconds (as low as 550 ps) may produce greater tensile stress on melanosomes than nanosecond pulse durations, enhancing their photomechanical and photothermal destruction. Collateral tissue heating and associated adverse events are minimized owing to the lower fluences required. As a result, potentially all skin types may be treated with this device. A 3- to 5-mm spot size and 1.5 to 2.83 J/cm 2 fixed fluence are favored.
Q-switched Nd:YAG lasers
With a wavelength of 1064 nm, these devices allow for much deeper energy penetration and minimal melanin absorption compared with QSRL or QSAL. Fitzpatrick skin types V and VI can thereby be treated with minimal risk of posttreatment dyspigmentation. The Spectra (Lutronic, Inc, Fremont, CA) 1064-nm Q-switched neodymium-doped Nd:YAG laser uses a collimated hand piece to deliver a high peak power over very short pulse durations (≤10 ns), maximizing selective photothermolysis of cutaneous melanosomes. As demonstrated in treating melasma, repeat sessions with low-fluence, Q-switched Nd:YAG treatments can decrease stage IV melanosomes, damage melanocytes, and reduce expression of melanogenesis-associated proteins. Greater fluences (4 to 5 J) can be used with a 3-mm spot size for other types of lower eyelid hyperpigmentation.
Thirty female Chinese subjects with under-eye circles owing to dermal melanin deposition received 8 low-fluence treatments (3.5-mm spot size, 4.2 J/cm 2 , 2 passes) at 3- to 4-day intervals. Blinded evaluators rated a mean global improvement of 50% to 75% at 3 and 6 months, and 93.3% subjects reported good to excellent results without significant adverse events.
Frequency-doubled Nd:YAG or potassium-titanyl-phosphate lasers (532 nm) can also effectively target pigmentation with nanosecond pulse durations. A split-face study of 10 female subjects with bilateral acquired nevus of Ota-like macules compared 532 nm with a combination of 532/1064 nm. Parameters included a 4-mm spot size with 1.2 J/cm 2 (532 nm) and 4 mm with 6.5 J/cm 2 (1064 nm). Objective measures of pigmentation and subject- and blinded physician-graded improvement were significantly better with combination treatment at 6 months after a single session.
Pulsed-Dye Lasers
Unlike prior flashlamp-pumped pulsed-dye lasers with short pulse durations (0.1 to 0.45 ms) and 577-nm light emission, current pulsed-dye lasers deliver 585 and 595 nm wavelengths over extended pulse widths (less than or equal to 40 ms) that allow for selective photothermolysis of larger, deeper ectatic vessels and a far greater purpuric threshold. Although pulse stacking and multiple passes at subpurpuric fluences with adequate epidermal protection (cryogen or convection cooling) lead to significant improvement in vessel clearance without added adverse events, multiple treatment sessions may still be needed. Superficial telangiectasias are treated with pulse durations and fluences of 6 ms and 7 to 9 J/cm 2 (less than 0.6 mm) or 10 ms and 8 to 12 J/cm 2 (greater than 0.6 mm) using a 7-mm spot size, with marginally overlapping pulses. Thicker facial vessels (∼1 mm) require 20 to 40 ms pulse widths and subpurpuric fluences as high as 13 to 15 J/cm 2 . One to 3 sessions at 4- to 8-week intervals are often needed. Diffuse erythema can be targeted with a 10-mm spot size and 6 or 10 ms at 5 to 8 J/cm 2 or 20 ms at 7.5 to 9 J/cm 2 . Dark-skinned patients should be treated with longer pulse durations and lower fluences. Treatment endpoint is immediate vessel spasm and transient purpura indicative of intravascular coagulation. Care should be taken when using cryogen cooling, because the cryogen is likely to enhance PIH.
Given that pulsed-dye laser wavelengths lie within the absorption coefficient for melanin, hyperpigmentation can be targeted with a 7-mm spot size using a single 10-ms pulse, low fluences (7 to 8 J/cm 2 ), and no epidermal cooling. Unlike the treatment of cutaneous vessels, pulse stacking or multiple passes should be avoided.
Long-Pulsed Nd:YAG Lasers
1064 nm Nd:YAG
Although dermal vessels strongly absorb 532 and 595 nm, approximating the absorption peaks of oxyhemoglobin, 1064 nm energy is poorly absorbed and requires significantly greater fluences (greater than 70 J/cm 2 ) for their thermocoagulation. Nevertheless, long-pulsed 1064 nm Nd:YAG devices are ideal for the treatment of larger, deeply situated facial vessels (eg, reticular veins) owing to the superior penetration of laser energy at this wavelength. Fitzpatrick skin types V and VI can be treated with low risk of epidermal injury, given the low absorption coefficient for melanin at 1064 nm.
The CoolTouch VARIA (CoolTouch, Inc, Roseville, CA) dispenses cryogen cooling before and/or after laser pulse delivery, maximizing treatment-related safety and reducing procedural discomfort. Treatment parameters for periorbital veins are based directly on vessel size and a 3.5-mm (range, 2 to 10) spot size; 1-mm reticular veins are treated with a 25-ms pulse duration and fluences of 160 to 190 J/cm 2 , whereas 1- to 3-mm veins require up to 50 ms and 190 to 210 J/cm 2 ( Fig. 6 ). Both have cryogen cooling of 20 to 30 ms delivered immediately after the laser pulse to quench transmission of heat from thermocoagulated blood vessels to the epidermis. A distal (superior) to proximal (inferior) technique ensures sufficient chromophore (hemoglobin) within subsequent areas of treatment. Vessel spasm or thrombosis is the endpoint of treatment, evidenced by immediate vessel blanching or darkening. Although pulse stacking or overlapping should be avoided to prevent bulk heating of treated areas, a second pass can be attempted after an interim of several minutes. One to 2 monthly sessions are required.