Cutaneous Melanoma: Introduction
|
Epidemiology
The incidence of melanoma has increased significantly worldwide over the last several decades. One in 50 born in the United States in 2010 is projected to develop invasive melanoma over their lifetime (1:39 men and 1:58 women), a 2,000% increase from 1930. With the inclusion of melanoma in situ, the lifetime risk increases to >1:30.1 It is estimated that 121,840 men and women were diagnosed with melanoma of the skin in 2009, of which 68,720 were invasive melanomas and 53,120 were melanoma in situ.2 Invasive melanoma of the skin is the fifth most frequent site for cancer to occur in men and the sixth most frequent site in women, representing approximately 5% of all newly diagnosed cancers.
Mortality rates for melanoma have recently stabilized for women, but continue to increase for men. The US surveillance, epidemiology, and end results (SEER) data estimates that in 2009, there were 8,650 deaths (5,550 men and 3,100 women) due to melanoma.2 Melanoma accounts for 75% of all skin cancer deaths. Melanoma is one of the leading cancers in terms of average years of life lost per death from disease.
The mean age of diagnosis is relatively young at 52 years, which is 10–15 years earlier than the mean age of diagnosis in the more common tumors of the breast, lung, colon, and prostate. More than 35% of melanomas occur in persons less than 45 years of age.1 Melanoma is the most common type of cancer in young adults in the US ages 25–29, second most common cancer in adolescents and young adults 15–29 years old. Incidence rises with age, especially in men. In the United States, women have a slightly higher incidence of melanoma than men before the age of 40 years. After age 40, men have a higher incidence, and the difference becomes remarkably large with increasing age. Mortality data parallel incidence data, with older men having the highest mortality rates. Although mortality rates in older persons, especially men, are rising, melanoma mortality rates in younger persons and females have been steady or actually declined despite a rising incidence of melanoma in young women.2–4 Lightly pigmented Caucasians have the highest incidence rates, much higher than Hispanics, Asians, and African-Americans.1
Etiology and Pathogenesis
|
Both genetic and environmental factors are related to melanoma pathogenesis and certainly not all melanomas are sun related. There is, however, clear convincing evidence that sun exposure, and more specifically ultraviolet (UV) exposure, is a major environmental cause of melanoma, especially in high-risk populations. Epidemiologic studies suggest that periodic, intense sun exposure (particularly during the critical time period of childhood and adolescence) rather than long, continued, heavy sun exposure is most important in melanoma causation, termed the intermittent exposure hypothesis. Sunburn history, notably blistering and peeling burns, serves as a surrogate measure of intermittent intense sun exposure. In one review of published literature, there was a significant positive association between sunburns during childhood and risk of melanoma development.5 One blistering sunburn in childhood more than doubles a person’s chances of developing melanoma later in life. Sunburns in adulthood have also been shown to contribute to melanoma risk.6 Data from the behavior risk factor surveillance system of the Centers for Disease Control and Prevention show that nearly 32% of all adults aged ≥18 years, 58% between 18 and 29 years of age, and more than 40% of children report having had at least one annual sunburn defined as red skin for more than 12 hours.7 Crude sunburn prevalence rates in white adults by state demonstrated higher sunburn rates among Caucasian adults living in the Midwest, possibly a reflection of a population that is more sun sensitive or less likely to practice sun-safe behaviors.
Migration studies generally indicate an increased melanoma risk in individuals who spent childhood in sunny geographic areas or emigrated to sunnier areas.8 Younger migrants to sunny areas have an increased risk for melanoma as compared with adult immigrants. Lastly, there is an increased melanoma risk with longer duration of residence in the sunny locale.
Melanoma incidence and mortality among Caucasians correlate inversely with latitude of residence and dose of UV radiation, termed the latitude gradient. The highest rates are nearest the equator. In areas as geographically diverse as the United States, New Zealand, and Australia, the incidence of melanoma is greater in regions closer to the equator. However, this gradient may be confounded by other risk factors for melanoma. In Italy, for example, more darkly pigmented persons reside in the South whereas more lightly pigmented persons live in the North, so that the latitude gradient is actually reversed.9 Even in the United States, SEER data from 1992 to 2001 demonstrate that the latitude gradient applies only to non-Hispanic whites; melanoma incidence was not associated with latitude and UV index in African-Americans, Hispanics, Asians, and Native Americans.10
The anatomic distribution of melanoma by body site demonstrates that intermittently exposed skin areas have the highest rates of developing melanoma. In men, the trunk, particularly the upper back, is the most common site for melanoma. In women, the lower legs, followed by the trunk, are the most common sites.11 These intermittently exposed areas are the most common areas to develop melanoma in younger persons. In older persons, there is a greater incidence of melanomas located on chronically exposed areas with maximal cumulative sun exposure. The face is the most common location for melanoma in older persons, with the addition of the neck, scalp, and ears as well, in older men.12,13
Several forms of artificial light have been associated with the development of melanoma, particularly psoralen and UVA light (PUVA) and UVB, and tanning booths. The so-called PUVA Follow-up Study demonstrated increased rates of melanoma after PUVA exposure, with an incidence rate ratio of 9.3 approximately 20 years after PUVA therapy; these rates increased over time and were higher in patients exposed to high cumulative doses of PUVA.12–14 However, the role of PUVA in the development of melanoma has been disputed on statistical grounds.15 Furthermore, an increased incidence of melanoma was not seen in a large follow-up study of PUVA-treated patients in Sweden, although a difference in treatment regimens between the United States and Sweden represented a potential confounding variable. There is also rising concern over tanning beds and melanoma risk, especially as exposure to the artificial UV radiation is intermittent in nature. Any exposure to artificial tanning devices modestly, but significantly, increases the risk of cutaneous melanoma (odds ratio, 1.25), and longer duration of bed use, younger age at first exposure, and higher frequency of use are associated with a significantly elevated risk (odds ratio, 1.69).16 Exposure to tanning beds early in life, before age 35, may be the most harmful pattern of tanning bed use, with a summary relative risk of developing a melanoma of 1.75.17 The evidence does not support a protective effect of tanning bed use before sun exposure.17
Light skin pigmentation, blond or red hair, blue or green eyes, prominent freckling tendency, and tendency to sunburn with Fitzpatrick skin phototype I–II are phenotypic features associated with an increased risk of melanoma.18–22 Melanoma occurs much less frequently in type V–VI skin, suggesting that skin pigment plays a protective role.
There is an increased risk of melanoma associated with nevi, both in a quantitative (i.e., number of nevi) and qualitative (i.e., typical vs. atypical nevi) manner.23–26 Adults with more than 100 clinically typical-appearing nevi, children with more than 50 typical-appearing nevi, and any patient with atypical nevi are at risk. The presence of a solitary dysplastic nevus may double the risk of melanoma, while having ten or more atypical nevi may be associated with a 12-fold elevation of risk.26 Nevi more often serve as a genetic marker of increased risk rather than a premalignant lesion, as most melanomas arise de novo. In a study of 1,606 patients with melanoma, only 26% of the melanomas were histologically associated with nevi (43% of these atypical nevi, 57% other nevi).27
On the other hand, large congenital nevi are recognized potential precursors of melanoma, although the degree of risk varies depending on the size of the lesion.28 Many series define large congenital nevi as greater than 20 cm in diameter in adulthood, and lifetime risks for developing melanoma are generally accepted to be in the 5%–10% range. It is estimated that 70% of melanomas in large congenital nevi develop before the age of 10 years old and may occur deep within the nevus or even in the central nervous system, making detection of a thin lesion difficult. Patients with large congenital nevi located on the posterior axis (paraspinal, head, and neck regions) or in conjunction with multiple satellite lesions are at risk for neurocutaneous melanosis, with an increased risk of developing melanoma in the central nervous system. For small- to medium-sized congenital nevi, the melanoma risk is similar to any other area of skin; in this case, melanoma usually occurs later in life (after puberty), and arises at the dermal-epidermal junction, making early detection possible.28 Thus, prophylactic excision of small- and medium-sized congenital nevi is usually unwarranted.
Patients with familial melanoma are estimated to account for 10%–15% of all patients with melanoma. Having one first-degree relative with melanoma doubles the risk of melanoma, whereas having three or more first-degree relatives with melanoma increases the risk 35- to 70-fold.29 Some of this risk may be attributed to shared risk factors such as skin phenotype, multiple nevi, and excessive sun exposure. The association between familial melanoma and multiple atypical nevi has historically been given various names, including B-K mole syndrome, familial atypical multiple mole-melanoma syndrome, and dysplastic nevus syndrome. Patients with familial melanoma typically have earlier onset melanoma and multiple primaries as well as atypical nevi. The molecular basis for some familial atypical multiple mole-melanoma syndrome kindreds are discussed under Section “Genetics.”
A previous history of melanoma increases the risk for another primary melanoma, with 5%–15% of individuals developing multiple primary melanomas.30,31 In patients with multiple primary melanomas, roughly one-half develop a second primary tumor in the same region of the body (i.e., trunk, extremity, head, and neck) and roughly one-half develop a second primary melanoma within the first year of initial diagnosis.30 However, subsequent primary melanomas may develop decades after diagnosis of the initial lesion, stressing the need for long-term surveillance. Subsequent melanomas are thinner compared with the first melanoma in roughly 70%–75% of cases.30
An association with an increased incidence of melanoma with immunosuppression remains unclear; ranging from no increased risk to a modest 1.5- to fivefold increased incidence in few studies.9,32 Several recent large reviews have revealed little to no increased risk of melanoma among HIV patients and no correlation of melanoma risk with declining CD4 count or the diagnosis of AIDS.32,33 Several studies have provided some evidence for an increased risk of melanoma in patients with a history of non-Hodgkin’s lymphoma (NHL), and possibly for melanoma patients having an increased risk of developing NHL, but these findings are not seen in all populations, and the clinical significance of these studies is not clear.34–36 Outcomes in immunosuppressed transplant recipients are reported as similar to matched nonimmunosuppressed patients, albeit in relatively small series.
Finally, a history of actinic keratosis or nonmelanoma skin cancer also confers a small increased risk of developing melanoma.
Germline mutations in the chromosome 9p21 tumor suppressor gene, cyclin-dependent kinase inhibitor 2A (CDKN2A), account for approximately 40% of hereditary melanoma cases (≥3 melanomas in one lineage) and confer a 76% chance of developing melanoma in the United States37; concordant with ambient rates, this risk is somewhat higher in Australia and lower in Europe. Individuals with germline CDKN2A mutations also exhibit a higher risk of pancreatic cancer; an estimated 15% of individuals with a mutant allele will develop pancreatic cancer in their lifetime.38 Bonafide deleterious point mutations in CDKN2A are relatively uncommon in primary melanoma tumors although homozygous deletions of this gene may obscure the true rate of somatic loss in melanoma. CDKN2A encodes two gene products: p16 (also known as INK4a, inhibitor of kinase 4a) and p14ARF (alternative reading frame). p16 is a cell-cycle regulator that binds and inhibits cyclin-dependent kinases Cdk4 or Cdk6, thereby inhibiting progression of cells through the G1 phase of the cell cycle. If p16 function is absent or inactivated by mutation, unrestrained Cdk4 activity phosphorylates the retinoblastoma protein thereby releasing the transcription factor E2-F and inducing S-phase entry. This sequence culminates in enhanced cellular proliferation, which, in the absence of checkpoint regulation, results in unrestrained growth and neoplasia.
The binding partner of the p16 protein is Cdk4. Only a handful of families worldwide thus far have been reported to carry hereditary mutations in CDK4 while somatic mutations in this gene have also been detected in some melanoma cell lines. Functional studies suggest that mutations in Cdk4 render the cyclin-dependent protein kinase resistant to p16 inhibition, resulting in a phenotype identical to that from p16 loss.
The p14ARF protein from CDKN2A inhibits a cellular oncogene Hdm2, which in turn accelerates the destruction of the p53 tumor-suppressor gene.39 Thus, complete loss of CDKN2A also leads to abrogation of p14ARF and loss of p53 function. Thus, this single locus can inactivate both the retinoblastoma protein and p53 pathways and probably explains the low rate of direct TP53 mutagenesis in melanoma.
Davies et al. first reported in 2002 a somatic mutation in the BRAF gene in 66% of melanomas.40 B-raf is a serine/threonine kinase, which is a major player in the Ras-Raf-Mek-Erk mitogen-activated protein kinase (MAPK) signaling transduction pathway that regulates cell growth, proliferation, and differentiation in response to various growth factors, cytokines, and hormones. The stimuli activate the G-protein Ras by inducing the exchange of guanosine 5′-diphosphate for guanosine 5′-triphosphate, which then binds and activates Raf. Raf phosphorylates and activates Mek, which in turn phosphorylates MAPK (i.e., Erks). This signaling cascade serves to intracellularly amplify the extracellular signals mediated by growth factors. There are three functional Raf proteins in humans, A-raf, B-raf, and C-raf. B-raf has a much higher basal kinase activity than either A-raf or C-raf, and somatic mutations in B-raf occur with moderate to higher frequency in melanoma and colorectal, ovarian, and papillary thyroid carcinomas, implicating activating oncogenic mutations of B-raf as critical promoters of malignancy.41
BRAF mutations are significantly more common in melanomas occurring on skin subject to intermittent sun exposure, with 81% of melanomas on skin without chronic sun damage having BRAF or NRAS mutations.42 On the other hand, the majority of melanomas from acral sites (palms, soles, and subungual skin), mucosa, or chronically sun-damaged skin do not carry a mutation in either gene. There are also significant differences in the number of copies of genomic DNA in melanomas located in the various regions. Melanomas with wild-type BRAF or RAS frequently had increases in the number of copies of the genes for CDK4 and CCND1 (cyclin D1), two critical G1 cell-cycle proteins and two genes that are transcriptionally induced by activated MAPK signaling.42
Mutations in PTEN/MMAC1 tumor suppressor gene also lead to enhanced Ras pathway signaling.43 This protein/lipid phosphatase downregulates signaling through the Akt arm of the Ras circuitry. When PTEN is inactivated, there is dysregulated Akt activity along with increased cellular survival. There is both genetic43 and animal data44 to support cooperativity between PTEN loss and B-raf activation in driving melanoma formation.
Recently, somatic amplifications of the microphthalmia (MITF) gene in a subset of melanoma tumors were identified, and it was demonstrated that overexpression of MITF, in conjunction with ectopic expression of BRAF, resulted in melanocyte transformation.45 The Mitf protein is a transcription factor that appears to be a master regulator of melanocyte differentiation, and amplification of this gene appears to contribute to a novel carcinogenic mechanism known as lineage addiction.
The KIT tyrosinse kinase receptor has also been shown to be mutated or amplified in a subset of melanomas predominantly from acral and mucosal sites.46 These oncogenic lesions occur mostly in the juxtamembrane domain (KIT exons 11, 13, and 17)—a region that is frequently targeted in other cancers including gastrointestinal stromal tumors.47 There is early evidence that imatinib, a tyrosine kinase inhibitor, may be useful in the treatment of c-Kit mutated melanomas.48
As mentioned above, red hair and sun sensitivity represent epidemiologically demonstrated risk factors for cutaneous melanoma. Recently, mutations in the melanocortin-1-receptor (MC1R) were shown to strongly contribute to the red hair/fair skin phenotype.49 As such, germline mutations in the MC1R gene have also been shown to increase melanoma risk by approximately two- to fourfold in the general population50; it also confers risk for nonmelanoma skin cancer. Because the prevalence of MC1R mutations is quite high in the white population, its attributable risk to melanoma is higher than the rarer high-risk CDKN2A mutations.
Xeroderma pigmentosum (XP) is a rare autosomal recessive genodermatosis comprised of at least seven complementation groups each defined by a separate gene. The XP genes are involved in excising DNA photoproducts in a reparative program termed nucleotide excision repair. Thus, heritable defects in these genes lead to increased mutagenesis and early carcinogenesis. Patients with XP harbor a 600- to 1,000-fold increased risk for skin cancer, including cutaneous melanoma.
Carriers of the breast cancer susceptibility gene, BRCA2, appear to harbor an increased risk for melanoma (2.58-fold).51 Although an interaction between breast cancer and melanoma risk has been suggested on epidemiologic grounds, the genetic interaction between BRCA2 and melanoma risk remains to be fully determined.
![]() Several genome-wide association studies (GWAS) have identified SNPs that tag risk conferring chromosomal regions. In one study from Iceland, investigators found evidence for risk SNPs around various pigmentation-associated loci: SLC24A4 (solute carrier family 24), KITLG (KIT ligand), 6p25.3, TYR (tyrosinase), OCA2 (oculocutaneous albinism II), TPCN2 (two-pore segment channel 2), ASIP (Agouti signal protein), and TYRP1 (tyrosinase-related protein 1).52 Studies from GenoMEL—the International Melanoma Genetics Consortium—have also independently identified risk-conferring SNPs on 20q11,53 11q14 (TYR) and 9p21 (CDKN2A–MTAP).54 The emerging picture from these GWAS analyses is that sporadic melanoma risk is still heavily modulated by pigmentary status and probably sun exposure. Regardless, the level of risk associated with these SNPs is often under twofold and the biological mechanisms underlying this predisposition are still unknown.
Several genome-wide association studies (GWAS) have identified SNPs that tag risk conferring chromosomal regions. In one study from Iceland, investigators found evidence for risk SNPs around various pigmentation-associated loci: SLC24A4 (solute carrier family 24), KITLG (KIT ligand), 6p25.3, TYR (tyrosinase), OCA2 (oculocutaneous albinism II), TPCN2 (two-pore segment channel 2), ASIP (Agouti signal protein), and TYRP1 (tyrosinase-related protein 1).52 Studies from GenoMEL—the International Melanoma Genetics Consortium—have also independently identified risk-conferring SNPs on 20q11,53 11q14 (TYR) and 9p21 (CDKN2A–MTAP).54 The emerging picture from these GWAS analyses is that sporadic melanoma risk is still heavily modulated by pigmentary status and probably sun exposure. Regardless, the level of risk associated with these SNPs is often under twofold and the biological mechanisms underlying this predisposition are still unknown.
Five stages of malignant transformation and tumor progression in melanocytes have been suggested, based on clinical, histopathologic, immunopathologic, cytogenetic, and in vitro properties: (1) benign melanocytic nevi; (2) atypical nevi; (3) primary malignant melanoma, radial growth phase; (4) primary malignant melanoma, vertical growth phase; and (5) metastatic malignant melanoma. It is believed that with each successive step of tumorigenesis, a new clone of cells emerges with growth advantages over the surrounding tissue, resulting in “clonal expansion.” It has been postulated that a critical step in tumor progression of melanoma may be the transition from radial to vertical growth phases. The radial growth phase consists of primarily intraepidermal proliferation of melanoma cells, but also invasion of the papillary dermis by small numbers of cells that have gained a growth advantage. These cells are thought to have the capacity for autonomous proliferation in this location, but not for aggregative growth. Radial growth phase cells are characterized by the presence of E-cadherin, an adhesion molecule that interacts with keratinocytes and impedes migration of the cells from their intraepidermal location.55 Melanomas in this phase are less capable of metastasis. The vertical growth phase is signaled by the property of aggregative growth, resulting in the formation of expansile nests or nodules of cells. Because the metastatic cascade comprises a complex range of numerous biochemical events, the vertical growth phase is likely an oversimplified correlate for the metastatic phenotype. Among other characteristics, vertical growth phase cells lose E-cadherin and express N-cadherin, a molecule that interacts with fibroblasts, macrophages, and endothelial cells. This latter interaction may facilitate intravasation of the malignant cells.55
A discussion of tumorigenesis of melanoma must take into account several important clinical observations: (1) the association between precursor nevi and melanoma in approximately 30% of cases (an apparent absence of precursor nevi in 70% of melanoma); (2) the role of UV light in the pathogenesis of melanoma; (3) pigmentary phenotype of patients in whom melanoma develops; and (4) family history of melanoma and other genetic factors. When the above observations are considered in light of the multistep process of tumor progression of melanoma, at least two major pathways of tumorigenesis can be envisioned.
In the first pathway, melanomas, particularly superficial spreading melanomas (SSMs), at least in some cases, develop in association with melanocytic nevi. Nevi may represent the first stage of tumor progression of melanoma or an initiated clonal proliferation. The most likely initiating agent would be UV exposure, perhaps at an early age. There is epidemiologic evidence that sun exposure earlier in life may result in larger numbers of nevi. The number of precursor nevi may be important as greater numbers of initiated cells would increase the target population for another mutational event. As UV is believed to be a complete carcinogen, sunlight exposure, perhaps of intermittent nature, could lead to this second mutational event, resulting in tumor progression. A second pathway of melanoma development is exemplified by lentigo maligna melanoma (LMM). This form of melanoma results from cumulative sun exposure and a corresponding cumulative insult to the DNA of melanocytes on sun-exposed skin. The age-incidence rates show a steady increase with age, consistent with continuous exposure (initiation) to a carcinogenic agent such as UV light.
Curtin et al. compared genome-wide alterations in the number of copies of DNA and mutational status of BRAF and NRAS in melanomas from variable groups in which the degree of exposure to UV light differed.42 The genetic alterations identified in melanomas at different sites with different levels of sun exposure indicated that there are distinct genetic pathways in the development of melanoma. This supports the hypothesis that the clinical heterogeneity notable for melanoma may be explained by genetically distinct types of melanoma with different susceptibility to UV light.42 Certainly, other as yet unknown pathways may exist that are independent of UV light.
Clinical Findings
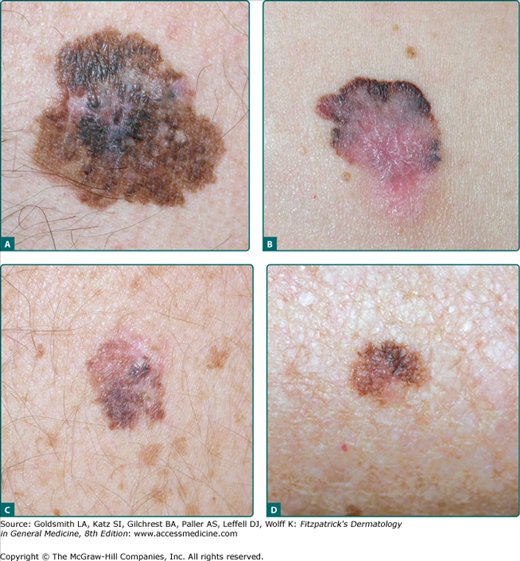
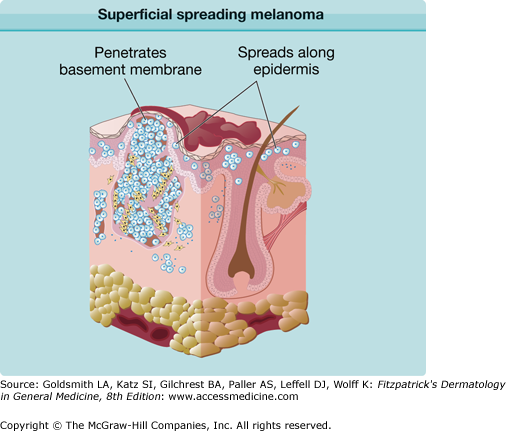
SSM is the most common subtype, accounting for approximately 70% of all cutaneous melanomas. It is diagnosed most commonly on intermittently sun-exposed areas, most frequently the lower extremity of women, and the upper back of men. Its classic clinical appearance best fits into the ABCD criteria (see Section “Making a Diagnosis”), with irregular borders and irregular pigmentation, but it may present subtly as a discrete focal area of darkening within a preexisting nevus. The range of appearance of SSM is broad (Figs. 124-1 and 124-2). Although varying shades of brown typify most melanocytic lesions, striking aspects of dark brown to black, blue-gray, pink, red, and gray-white (which may represent regression) may be found in melanoma. SSM is the subtype of melanoma most commonly associated with preexisting nevi. The history of SSM is often of a lesion slowly changing over months to years. It may be mistaken for an atypical nevus or seborrheic keratosis.
Figure 124-1
Superficial spreading melanoma (SSM). A. Classic clinical appearance with cardinal features of SSM: asymmetry, irregular scalloped borders, mottled variegate color, large diameter, and central elevation with surface distortion in a 45-year-old man, left chest, Breslow depth 1.25 mm. B. Another SSM with similar features but with pronounced areas of pink, shades of blue and white, with peripheral areas of black and brown in a 54-year-old woman, right lower abdomen, Breslow depth 3.5 mm, 26 mitoses/mm2, ulceration focally present. C. Earlier detection in a 55-year-old man with many atypical nevi, patient unaware of the lesion, lesion noted by primary care physician during a routine physical examination, “different” than other nevi, right upper back, Breslow depth 0.50 mm. The pink/tan upper portion is an atypical nevus, the lower portion SSM arising in an atypical nevus. D. Still earlier detection with more subtle asymmetry, border, color, diameter/difference, elevation/evolving (ABCDs), forearm, Breslow depth 0.14 mm. The lesion was 0.8 cm in diameter with a history of a “brown spot,” which grew in size, changed shape, and changed to a darker color over several months.
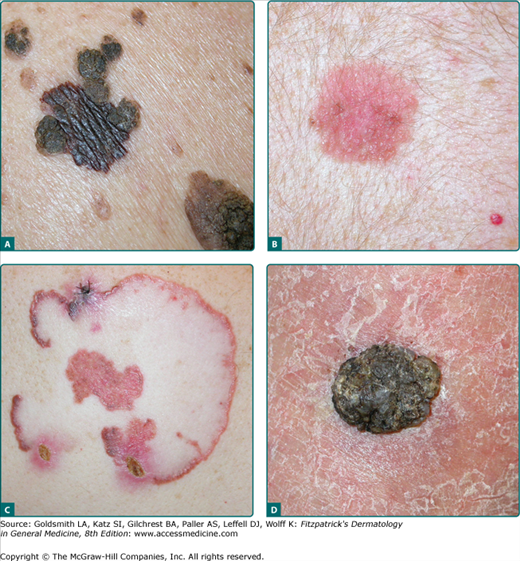
Figure 124-2
Superficial spreading melanoma (SSM) showing a broad range of appearance. A. Breslow depth 0.51 mm, central back within a “sea” of seborrheic keratosis but “different” than the surrounding seborrheic keratoses. B. Amelanotic SSM, upper back, Breslow depth 0.38 mm. C. Unusual presentation, left posterior shoulder, 60-year-old man, 11 cm × 9 cm SSM, slowly growing peripherally for over a decade, large areas of regression, pink amelanotic areas, and a small black component in the superior pole, Breslow depth 1.60 mm, 3 mitoses/mm2. D. Unusual presentation of SSM with papillomatous features, right ankle, Breslow depth 6.3 mm, 3 mitoses/mm2, vertical growth phase.
(See Box 124-1)
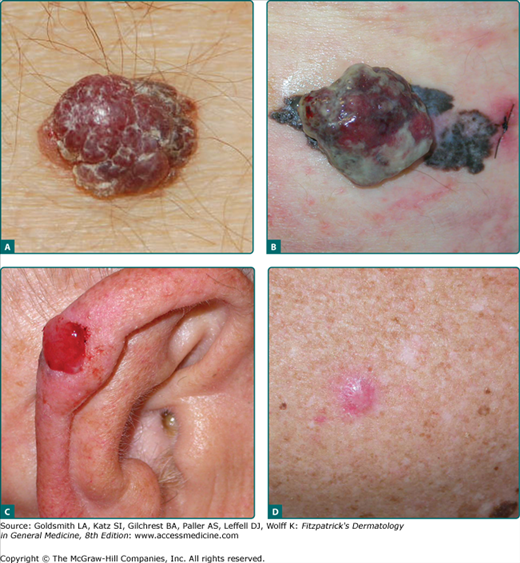
Nodular melanoma (NM) is the second most common melanoma subtype and accounts for approximately 15%–30% of all melanomas. The trunk is the most common site. NM is remarkable for rapid evolution, often arising over several weeks to months. NM more often lacks an apparent radial growth phase. It is more common for NM to begin de novo than to arise in a preexisting nevus. NM typically appears as a uniformly dark blue-black or bluish-red raised lesion, but 5% are amelanotic (Fig. 124-3). A substantial proportion of thick melanomas are of the nodular type.56 Early lesions often lack asymmetry, have regular borders, and are a uniform color. Amelanotic lesions may be mistaken for basal cell carcinoma, pyogenic granuloma, or hemangioma, whereas pigmented lesions may be mistaken for blue nevi or pigmented basal cell carcinomas.
Figure 124-3
Nodular melanoma (NM). A. Classic NM, left lower back, Breslow depth 4.3 mm, angiolymphatic spread present. (Used with permission from Walter Barkey, MD.) B. Nodular polypoid melanoma, left flank, Breslow depth 16.0 mm, 38 mitoses/mm2, ulceration and angiolymphatic spread present. C. Amelanotic NM, Breslow depth 2.37 mm, 32 mitoses/mm2, vertical growth phase, ulceration present. D. Amelanotic NM with spindle cell features, noted approximately 1–2 months with elevation and bleeding, right posterior shoulder, Breslow depth 3.95 mm, 6 mitoses/mm2.
(See Box 124-2)
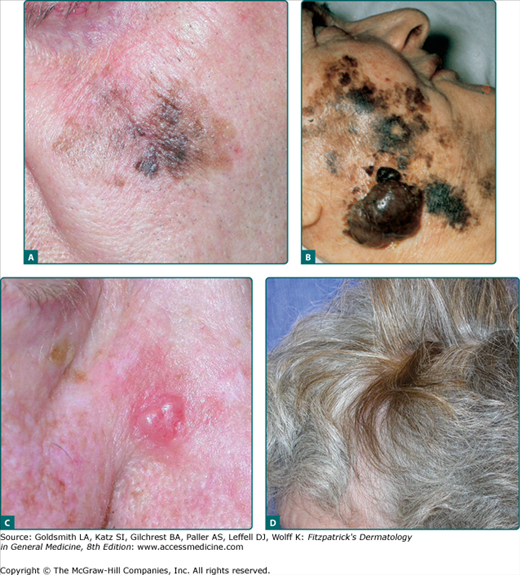
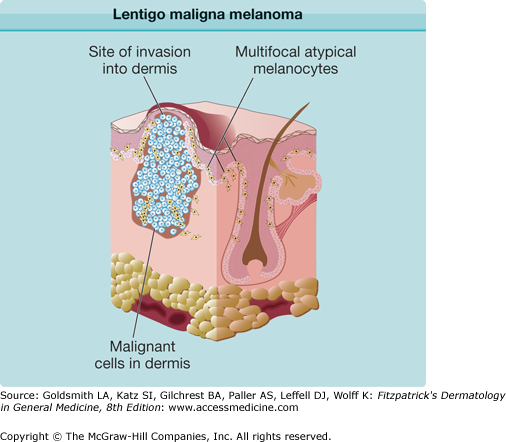
LM is a subtype of melanoma in situ with a prolonged radial growth phase that may progress to invasive LMM with time. Invasive LMM constitutes 10%–15% of cutaneous melanomas. LM and LMM are diagnosed most commonly in the seventh to eighth decades in an older population than other types of melanoma; uncommon before the age 40. The most common location is on the chronically sun-exposed face, on the cheeks and nose in particular; the neck, scalp, and ears in men. Its pathogenesis is thought to be related to cumulative sun exposure rather than intermittent exposure. LM is a flat, slowly enlarging, brown, freckle-like macule with irregular shape and differing shades of brown and tan, usually arising in a background of photodamage (Fig. 124-4). The lifetime risk of LMM developing from LM is estimated to be low but the invasive component is potentially lethal.57 LMM is frequently larger than LM and may continue to be macular in early lesions, although a nodular portion is often seen within the macule later. Both LM and LMM often have clinically ill-defined borders, which may be obscured by background actinic damage consisting of lentigines, pigmented actinic keratoses, or ephelides. LM and LMM are associated with significantly higher rates of extensive subclinical lateral growth, resulting in higher recurrence rates with standard recommended margins and failure to completely excise the lesion. LM and LMM have the least common association with nevi, at 3% of cases, but the highest rate of association with desmoplastic melanoma (DM, see below).
Figure 124-4
Lentigo maligna and lentigo maligna melanoma (LMM). A. Lentigo maligna (in situ) displays prominent asymmetry, poorly defined irregular borders, and pigment variegation. B. Extensive LMM with large nodules. C. Amelanotic LMM with nodular component initially suspected to be a basal cell carcinoma, nose, Breslow depth 2.2 mm, 5 mitoses/mm2, ulceration present. D. A pigmented patch of hair was noted after discontinuing practice of hair coloring. Over 6 weeks the area became pruritic and bled once. E. Inspection of the skin in the area of pigmented hair revealed the melanoma. Breslow depth 3.4 mm, ulceration present, 6 mitoses/mm2, two foci of dermal melanoma satellite metastases.
(See Box 124-3)
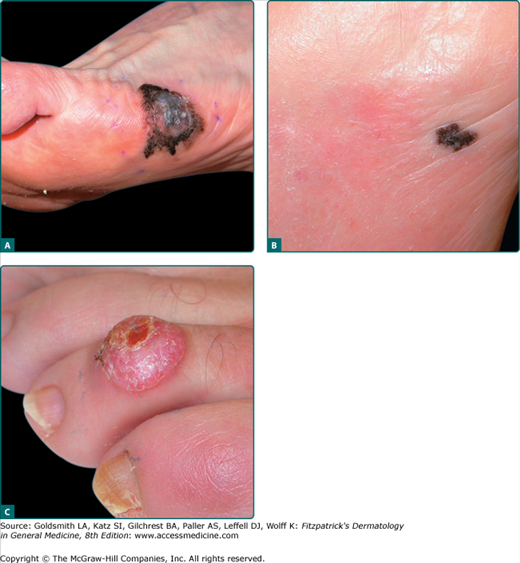
Acral lentiginous melanoma (ALM) is a subtype of melanoma with distinct differences in frequencies seen between ethnic groups. ALM constitutes only 2%–8% of melanomas in Caucasians but represents the most common form in darker-pigmented individuals (60%–72% African-Americans and 29%–46% Asians). Although the proportion of ALM seen in darker-pigmented individuals is higher, the incidence of ALM is similar for Caucasians and other ethnicities. ALM is diagnosed more often in an older population, with the median age of onset of 65. The most common site for ALM is the sole, with the palm and subungual locations following (Fig. 124-5). Not all palmar or plantar melanomas are ALMs; a minority are SSMs or NMs. The clinical appearance of ALM can be brown, black, tan, or red with variegations in color and irregular borders; however, the most common color is brown-black. There is often a delay in the diagnosis of ALM, often misdiagnosed as a plantar wart or hematoma, leading to a more advanced lesion upon diagnosis associated with poorer outcomes.58 ALM is not thought to be associated with sun exposure.
Figure 124-5
Acral lentiginous melanoma (ALM). A. Classic ALM demonstrating asymmetry, border, color, diameter/difference, elevation/evolving (ABCDs), left foot, Breslow depth 1.72 mm, ulceration present. B. Early detection of lesion followed by the patient, present for many decades, over several months began growing with irregular border and shape, changed in color from brown to black, in situ ALM arising in a dysplastic nevus. C. Amelanotic ALM, fourth toe of left foot, Breslow depth 5.0 mm.
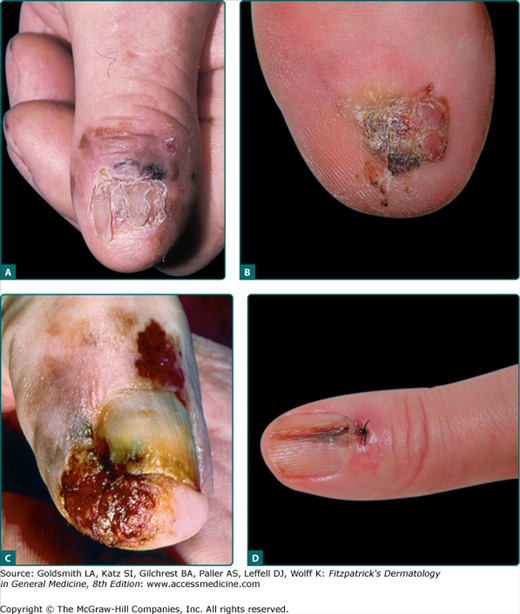
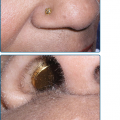
Subungual melanoma, considered a variant of ALM, generally arises from the nail matrix, most commonly on the great toe or thumb (Fig. 124-6). It appears as a brown to black discoloration or growth in the nail bed. A widening, dark, or irregularly pigmented longitudinal nail streak (melanonychia striata) with or without nail dystrophy and nail plate elevation may be seen. Hutchinson sign, the finding of pigmentation on the proximal nail fold, may be noted with subungual melanoma. Benign lesions that mimic subungual melanoma include: benign longitudinal melanonychia, subungual hematoma, or pyogenic granuloma or even onychomycosis with pigmentation or hemorrhage.
Figure 124-6
Subungual acral lentiginous melanoma (ALM). A. Extensive involvement of periungual skin (Hutchinson sign) with dystrophy and loss of the nail plate secondary to the tumor. B. ALM great toe, without notable Hutchinson sign, Breslow depth 3.4 mm, 6 mitoses/mm2, two positive sentinel nodes identified, disease free at 2 years. C. Advanced subungual ALM with proximal periungual macular component and subungual and distal ulcerated nodular component. D. Subungual ALM, thumb nail bed streak, Breslow depth 0.5 mm. This was her fourth primary melanoma, other three located on the shoulder, cheek, and neck, the first 14 years prior.
Differential Diagnosis
|
DM most commonly develops in the sixth or seventh decade on sun-exposed head and neck regions. The lesions typically have a firm, sclerotic, or indurated quality, and one-half are amelanotic. Approximately half of the lesions arise in association with the LM histologic subtype. The DM pattern subtype may be associated with a higher rate of local recurrence due to a propensity to infiltrate perineurally with neurotropism and failure to appreciate occult growth, which may be deeply invasive at diagnosis. Although deeply invasive, DM is associated with lower nodal metastatic rates than other subtypes of melanoma when matched for depth of invasion. A recent study has shown that lesions with fusiform and/or epithelioid melanoma cells had a higher metastatic rate than those with pure DM with only fibrosing component.59 Results from a small study that involved ten samples that investigated gene expression profiling demonstrated a molecular distinction between DM and nondesmoplastic melanoma.60
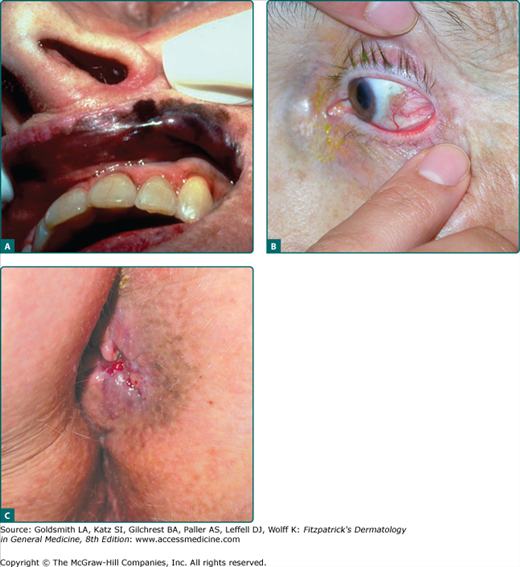
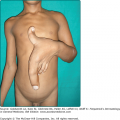
Melanoma can infrequently arise on mucosal surfaces on the head and neck (conjunctival, intranasal, sinus, and oral cavities), vulva, anorectal, or even urethral mucosa (Fig. 124-7). With the exception of the conjunctiva, patients present most often with delayed detection and a deeply pigmented, irregular lesion, but due to its location, may also present with bleeding or a mass lesion.61 As most of these lesions present with a radial growth phase manifesting a macular pigmentation, any suspicious area in these sites should be biopsied. Lesions of the conjunctiva are visible and appear increased in patients with atypical nevi.62,63
Nevoid melanoma describes a heterogeneous group of rare lesions that histologically resemble benign nevi by their symmetry and apparent maturation with descent in the dermis, thus with greater potential for misdiagnosis. Clues to their histologic diagnosis include marked hyperchromasia of the nuclei of the tumor cells, the presence of mitoses, and an expansile growth of the dermal cells with effacement of the adventitia in affected areas. Clinically this may correspond to a tan papule or nodule, more often >1 cm in diameter on a young adult.64
Spitzoid melanoma is a subtype of melanoma that clinically and histologically resembles a Spitz nevus, but tends to be larger and have asymmetry and irregular coloration. Distinction between the two is sometimes extremely difficult, and tumors with overlapping features of Spitz nevi and melanoma are classified as atypical Spitz tumors of uncertain biologic behavior (AST).65 Features that favor the diagnosis of a Spitzoid melanoma over a benign Spitz nevus are large lesions, greater than 1 cm in diameter; lesions with a thick invasive component, well over 2 mm; lesions with numerous mitoses, many atypical, and lesions that have clinically concerning course such as rapid growth in size or satellitosis. Ambiguous lesions that are not clinically or histologically diagnostic for Spitzoid melanoma nor benign Spitz nevi, but with overlapping features of both, may be classified as AST.65 Ongoing work with molecular profiling to identify genetic alterations in these tumors may provide markers to stratify AST into high- or low-risk lesions.
Several other rare and unusual types of melanoma are beyond the scope of this chapter, but previously reported in a comprehensive review, which include metaplastic melanoma, balloon cell melanoma, signet-ring cell melanoma, myxoid melanoma, rhabdoid melanoma, minimal-deviation melanoma, and animal-type melanoma, malignant blue nevus.64,66
Melanoma is the second most common cancer in women of child-bearing age, and thus constitutes one of the more common types of cancer diagnosed in pregnancy.9,67 Historically, controversy existed regarding the effect of pregnancy on the clinical course of melanoma. The basis of potential hormonal effects included hyperpigmentation associated with pregnancy and the elevation of endogenous hormones, including estrogens and melanocyte-stimulating hormone. However, the literature demonstrates that pregnancy does not increase the risk of developing melanoma.68 Furthermore, pregnancy before, during, or after a woman is diagnosed with melanoma does not appear to influence overall survival, although disease-free survival potentially may be shorted.69,70,71 There is no reported difference in the distribution or stage of disease, tumor thickness, lymph node positivity, or overall survival for pregnancy-associated melanoma (i.e., melanoma diagnosed during or within 1 year).72 Pregnant women with melanoma should be offered the same surgical treatments, including sentinel lymph node biopsy (SLNB) (usually with omission of blue dye) as nonpregnant patients; however, certain chemotherapeutic and immunologic regimens used in advanced melanoma may be contraindicated in pregnancy. After the initial diagnosis and treatment of melanoma, there are no standard, defined guidelines for patients who desire to become pregnant. Counseling recommendations should be comprehensive and individualized on a case-by-case basis, depending primarily on the risk of recurrence (related to the stage at diagnosis), patient age, and degree of personal desire to become pregnant relative to risk.
Melanoma in childhood and adolescence is rare. Of all newly diagnosed melanomas, 1%–4% occur in patients younger than 20 years of age, and only 0.3%–0.4% occur in prepubertal children.73 However, melanoma is being increasingly diagnosed in this age group and being reported to occur more commonly without the classic predisposing risk factors of familial melanoma, congenital nevi, dysplastic nevi, and xeroderma pigmentosum.74 Based on SEER data of 1,255 melanoma patients less than 20 years of age, a 2.9% increase in the incidence of pediatric melanoma per year from 1973 to 2001 has been noted; the rate of increase was lower (1.4%) in young children less than 10 years.75 Factors conferring risk of adult melanoma, including increasing age, UV exposure, and Caucasian background, were also found to be important in pediatric melanoma. The classic predisposing factors for adult melanoma are likely to be applicable to some proportion of children. In general, pediatric melanomas tend to have a greater proportion of thicker primaries, possibly related to delayed diagnosis compared with adults.
Early detection is the key to improving prognosis in melanoma. Although melanoma may have a characteristic appearance, there is no single clinical feature that ensures or excludes a diagnosis of melanoma. Even among expert dermatologists, the clinical diagnosis of melanoma can be made in about 80%–90% of cases. The well-known ABCD acronym for melanoma detection continues to be a useful tool for the lay public and physicians.76 A stands for asymmetry (one half is not identical to the other half); B for border (irregular, notched, scalloped, ragged, or poorly defined borders as opposed to smooth and straight edges); C for color (having varying shades from one area to another); and D for diameter (i.e., greater than 6 mm, approximately the size of a pencil eraser). Lesions having these characteristics may potentially represent melanoma. Studies have found the sensitivity of the ABCD checklist (Box 124-5) to be very high, but the specificity much lower.77 Another diagnostic aid that is useful in detecting melanoma is the “ugly duckling” sign.78 A pigmented lesion that is different from other pigmented lesions on a particular individual should be approached with a high index of suspicion. This is based on the premise that within an individual, nevi should globally share a common appearance or family resemblance. Even in an individual with multiple atypical nevi, the nevi should be morphologically similar.
History is very important in the evaluation of a lesion. The old ABCD rule is relatively static and does not account for a critical element of change. For this reason, some have added an E to the ABCD rule, where E stands for evolving.79 Others have advocated for the D to stand for “difference” (i.e., a change in appearance or symptomatology over time or difference from the other lesions, similar to the “ugly duckling” sign). Change in color and increase in size (or a new lesion) are the two most common early characteristics noticed by patients that may be useful in discriminating between melanoma and other benign lesions.80 In addition to change in color, size, or shape/elevation, persistent lesional itching is also an earlier (albeit nonspecific) symptom; ulceration, bleeding, and tenderness generally signify a more advanced primary lesion.81 Therefore, it is important to ask patients if lesions have changed over time, and to pay particular attention to changing or symptomatic lesions. The development of a new pigmented lesion, especially in an individual beyond the age of 40–50 years old and on an anatomic site without similar lesions, is also worthy of suspicion.
The skin examination should be conducted under optimal lighting and encompass the entire skin integument, including the scalp, external ocular/conjunctivae, oral mucosa, groin, buttocks, and palms/soles/web spaces. Melanomas in hidden anatomic sites are associated with thicker tumors at diagnosis, often due to later detection.82 A global assessment of an individual’s skin, including number of nevi, both typical- and atypical-appearing, and distribution of nevi should be noted. Other risk factors, such as a family or personal history of melanoma, skin type, UV history including tanning booths, or previously biopsied atypical nevi, should also be noted. Total body and lesional photography may be useful, especially in high-risk patients with numerous nevi.83 The use of photography to document the appearance of skin lesions (or the absence of a skin lesion) can allow for better and earlier detection of a changing or new lesion. In high-risk patients, a proportion of melanomas are diagnosed with the aid of photographic surveillance rather than being noted by the patient or even the physician.84 If a lesion suspicious for melanoma is found on skin examination, palpation of the regional lymph nodes should be performed to evaluate for lymphadenopathy.
Dermoscopy (also known as epiluminescence microscopy, dermatoscopy, incident light microscopy, and surface microscopy) is a noninvasive technique in which a handheld device is used to examine a lesion through a film of liquid, usually immersion oil, using nonpolarized light (contact dermoscopy), or the lesion is examined under polarized light without a contact medium (noncontact dermoscopy). In experienced hands, it may improve both the sensitivity and specificity for the clinical diagnosis of melanoma and other pigmented and nonpigmented lesions. Morphologic features that are otherwise not visible to the naked eye are observed using this technique.85
Different diagnostic algorithms using dermoscopic findings have been developed for melanoma, including the ABCD rule, the seven-point checklist, pattern analysis, Menzies method, modified ABC-point list, and CASH (color, architechture, symmetry, and homogeneity).86–91 Several studies have compared these methods to determine the most effective method for dermoscopic detection of melanoma.92–94 Pattern analysis, which provides an overall impression of multiple dermoscopic patterns without rigid rules, based primarily on a subjective, simultaneous evaluation of a number of different criteria, is the most widely used method among experienced users of dermoscopy for evaluating pigmented lesions.85 Lists of the important dermoscopy features with clinical pathologic correlations and significance have been widely published and are easily obtainable through courses, societies, and the Internet.
Digital dermoscopy or digital epiluminescent microscopy permits computerized digital dermoscopic images to be retrieved and examined at a later date so that comparisons can be made and changes detected over time. There are also a number of commercially available automated computer image analysis programs, devices that incorporate image analysis algorithms to digital dermoscopic images, providing objective measurement of changes over time. The use of automated instruments for the diagnosis of melanoma continues to evolve, but no system has demonstrated superior results to date in a clinically useful form. The use of body or lesional photography, professionally obtained or taken by the patient, is also useful to aid physicians and patients for following lesions, particularly in patients with many nevi. Finally, confocal scanning laser microscopy and multispectral digital dermatoscopy are among a number of new imaging techniques being evaluated for early detection of melanomas.95,96
The gold standard for diagnosing melanoma is based on histopathologic evaluation of the biopsy specimen by a dermatopathologist or pathologist experienced with pigmented lesions.97–99 The histologic diagnosis of melanoma is based on the assessment of a constellation of findings, including both architectural and cytologic features. No single feature is diagnostic. Cytologic atypia, referring to cellular enlargement, nuclear enlargement, nuclear pleomorphism, hyperchromasia of nuclei, nucleolar variability, and the presence of mitoses especially deep in the dermis, is considered necessary for a diagnosis of melanoma. The major architectural features of melanoma include asymmetry, poor circumscription (i.e., cells at the edge of the lesion tend to be small, single, and scattered), and large size (>5–6 mm). Nests of melanocytes in the lower epidermis and dermis tend to vary a good deal in size and shape, and to become confluent. There is a lack of maturation of nests with descent into the dermis. Pagetoid spread of large solitary epidermal melanocytes, usually considered diagnostic of melanoma, should be assessed cautiously in the context of other findings, as pagetoid spread may be seen in benign lesions including Spitz nevi, spindle cell nevi, vulvar nevi, and acral nevi.
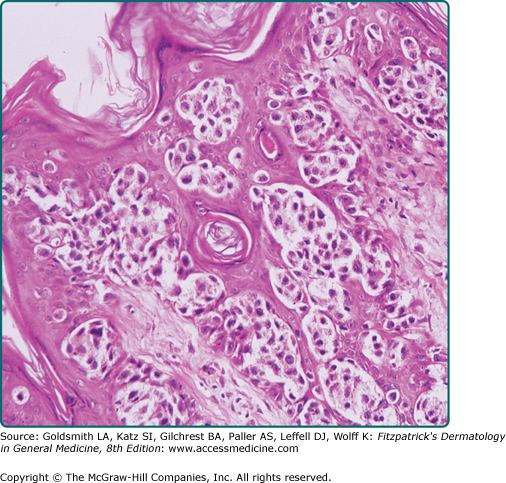
The different subtypes of melanoma have histopathologic differences as well. SSM is characterized by a population of melanocytes appearing uniformly atypical (Figs. 124-8 and 124-9). LM and LMM are characterized by atypical melanocytes singly and in nests, predominantly confined to the basal layer of the epidermis, which become confluent without pagetoid spread, occurring in the background of photodamage (Figs. 124-10, 124-11, and 124-12). The cells may extend down hair follicles and appendageal structures. The epidermis is thin and atrophic, with loss of rete ridges. There is variable cytologic atypia. The “lentiginous” in ALM is derived from the characteristic lentiginous pattern of most cells being single and located near the dermal-epidermal junction (Fig. 124-13). ALM differs from LM in that there is irregular acanthosis, and the melanoma cells are uniformly malignant and often dendritic. In LM, the cells are highly pleomorphic in an atrophic epidermis. NM demonstrates little tendency for intraepidermal growth; instead, there is a dermal mass of atypical melanocytes (Figs. 124-14 and 124-15). DM is composed of strands of elongated, spindle-shaped cells that often infiltrate deeply.
Figure 124-8
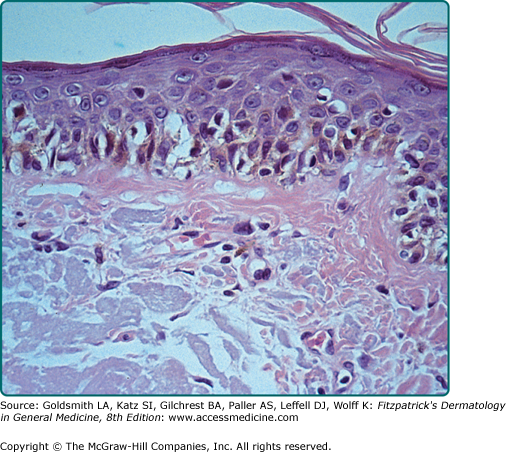
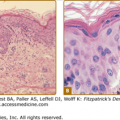
Superficial spreading melanoma. This tumor shows intraepidermal growth. In this photomicrograph, the pagetoid distribution is evident in the epidermis. The cells are relatively uniform and have an abundance of dusty, fine pigment. These relatively large melanoma cells are frequently referred to as epithelioid cell type.
Figure 124-9
Superficial spreading melanoma. The border is irregular and the lesion is elevated throughout its entirety. Biopsy of the area surrounding the large nodule shows a “pagetoid” distribution of large melanocytes that are occurring singly or in nests, and uniformly atypical. On the left is a large nodule, and scattered throughout the surrounding portion of the nodule are smaller papular and nodular areas. The nodule on the left shows malignant melanocytes that are very large, have an abundance of cytoplasm, and often have regularly dispersed fine particles of melanin. The nodules may also show spindle cells or small malignant melanocytes as in lentigo maligna melanoma and nodular melanoma.
Figure 124-10
Lentigo maligna melanoma. Illustrated on the right is a large, variegated, freckle-like macule (not elevated above the plane of skin) with irregular borders; the tan areas show increased numbers of melanocytes, usually atypical and bizarre, and are distributed single file along the basal layer; at certain places in the dermis, malignant melanocytes have invaded and formed prominent nests. At the left is a large nodule that is composed of epithelioid cells in this illustration; the nodules of all four main subtypes of melanoma are indistinguishable from each other.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree