Obstructive Peripheral Arterial Disease
|
Although atherosclerotic obstructive peripheral arterial disease (PAD) has a prevalence of only 3% in patients of age 40–59 years, this rises to 15% in the age group older than 65 years. This translates into approximately 9 million cases in the United States in 2005, and this number is expected to increase along with the aging demographics of our population.1,2 PAD is often unrecognized clinically and more than one-half of all patients are asymptomatic. Gender predisposition shows preponderance in males, although the incidence in females rises rapidly after menopause. Anatomically, superficial femoral artery disease predominates, with development of symptoms typically in the seventh decade. Interestingly, symptoms from aortoiliac disease usually present a decade earlier.
Atherosclerotic risk factors are similar to those identified for coronary artery disease and include diabetes mellitus, hypertension, hyperlipidemia, smoking, family history of vascular disease, and obesity. Among these, diabetes mellitus and smoking are the most significant and are associated with a fourfold and doubling of relative risk, respectively. Patients with diabetes mellitus develop the disease at an earlier age than nondiabetics and have more severe and progressive disease. The anatomic distribution of obstruction differs from nondiabetics with less aortoiliac involvement and more extensive disease of the runoff vessels below the knees; however, superficial femoral artery disease is similar in both populations. Approximately 50% of patients have hyperlipidemia. PAD is also more commonly encountered in patients with hypertension.
The pathologic findings in atherosclerosis occur in large- and medium-sized arteries, and are morphologically diverse, with focal accumulation of lipids and lipoproteins, mucopolysaccharides and collagen, smooth muscle cells and macrophages, and calcium deposits in variable quantities. Localized areas of intimal thickening secondary to smooth muscle cell proliferation and lipid-laden macrophages are seen in early stages with disruption of the internal elastic lamina. The media is often atrophic with thin strands of smooth muscle, lipid pools, collagen tissue, and calcium deposits. Enlarging plaques encroach on the lumen despite dilation of the artery, and the plaques may ulcerate. Hemorrhages occur in the arterial wall. Thrombi may form and occlude the narrowed arterial lumen.
The etiology of atherosclerosis is complex and multifactorial, but progressive buildup of plaque narrows the vessel lumen, and complete occlusion may develop acutely secondary to thrombosis. Because disease progression is usually over an extended time period, collateral blood vessels have time to develop and are usually robust. Tissue perfusion to the affected limb is often adequate at rest, but the blood pressure distal to the occlusions is decreased secondary to high resistance and limited flow through collateral vessels. Under resting conditions, normal blood flow to extremity muscle groups averages 300–400 mL/min. Once exercise begins, blood flow increases up to tenfold owing to the increase in cardiac output and compensatory vasodilation at the tissue level. When exercise ceases, the blood flow returns to normal within minutes. In patients with PAD, resting blood flow may be similar to that of a healthy person. However, during exercise, blood flow cannot maximally increase in muscle tissue because of fixed proximal arterial stenoses. When the metabolic demands of the muscle exceed blood flow, claudication symptoms ensue. At the same time, a longer recovery period is required for blood flow to return to baseline once exercise is terminated. Dermatologic changes imply severe compromise of tissue perfusion, often secondary to tandem high-grade stenoses or occlusions present at multiple levels in the arterial tree.
The most classical symptom of PAD is intermittent claudication, which is usually described as pain, fatigue, or tiredness in the muscles distal to the diseased vascular segment on walking. The location of the pain differs depending on the anatomical location of the arterial lesions. Since the disease is most common in the distal superficial femoral artery, patients most commonly present with claudication in the calf muscle area (the muscle group just distal to the arterial disease). When the disease affects the more proximal aortoiliac vessels, thigh and buttock muscle claudication predominates. The discomfort tends to be highly reproducible within the same muscle groups and is precipitated by the same level of exertion. It is crucial to determine the amount of walking distance before the onset of symptoms; this helps to quantify patients with some standard measure of walking distance before and after therapy. Resolution within several minutes of rest is an expected finding. Patients with inadequate collateral circulation may complain of cold extremities, hyperesthesia, rest pain, discolored toes, or skin breakdown. Ischemic rest pain typically affects the foot and may interfere with sleep or necessitate sleeping with the leg in a dependent position. Peripheral edema may then occur.
Acute limb ischemia secondary to vessel thrombosis or embolism presents more dramatically with (1) severe pain, usually persistent at rest, (2) pallor, (3) pulselessness, (4) paresthesias, and (5) paralysis (the five Ps). Poikilothermia (a cold extremity) may be added to this pentad of clinical findings (Box 173-1). The presence of neurologic symptoms indicates severe ischemia and need for emergent evaluation.
Since atherosclerosis is a systemic disease process, patients who present with claudication due to PAD can be expected to have atherosclerosis elsewhere. Hence, it is common for patients to present with symptoms related to disease affecting the other vascular territories.
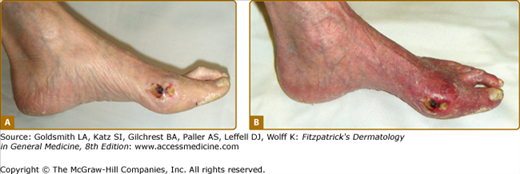
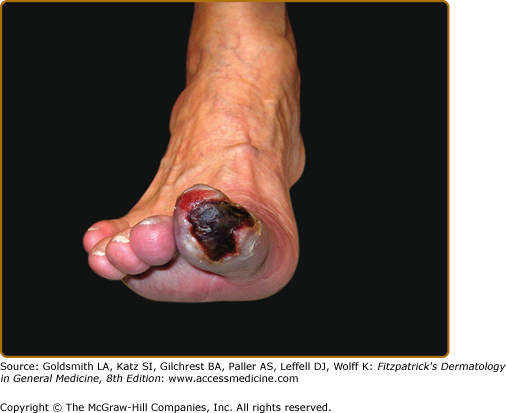
The cutaneous findings in PAD will vary depending on the severity of arterial obstruction and tissue ischemia (Table 173-1). Limbs in patients with intermittent claudication may appear normal, although associated clinical findings may include hair loss, coldness, and cyanosis, or thickened and malformed toenails. In patients with severe ischemia, the skin is apt to be atrophic, dry, and shiny. In patients with rest pain, the foot is usually bright red and cold in dependency. Ulcerations most often start at the tips of the toes or on the heel of the foot and are extremely painful except when diabetic neuropathy is also present (Fig. 173-2). Ulcers also occasionally start on the lower calves or lateral aspect of the heel. They frequently demonstrate irregular borders and a pale base. The heels may show cracks in the skin with numerous fissures. When gangrene occurs, usually one or more toes become black, dry, and mummified (Fig. 173-3). Superimposed infection is a major concern, and important associated signs include purulent discharge or decay (wet gangrene) or surrounding tissue erythema and swelling.
Figure 173-2
Obstructive peripheral arterial disease. An ulcer is seen on the medial aspect of the foot at the base of the great toe. A. Foot pallor with elevation of limb suggests severe ischemia. B. Foot erythema with the leg in a dependent position, termed dependent rubor, also suggests severe ischemia.
The hallmark characteristic is decreased or absent pulses distal to the stenotic arterial segment, and there may be bruits on auscultation over the diseased segment of vessel secondary to turbulent flow. It is also possible to find normal palpable pulses in a patient who presents with a history consistent with typical intermittent claudication. In such a case, the clinician can have the patient walk around the office (or perform toe raises) until the symptoms are reproduced and then palpate for pulses. The exercise may “unmask” a stenosis causing the atherosclerotic lesion to become significant and should diminish the strength of the pulses distal to the lesion. The collateral circulation to limbs affected by PAD can be evaluated by simple bedside exam. With the patient supine, elevation of the limb at a 45° angle for 2 minutes should not produce pallor. Collateral circulation is deemed inadequate if the toes and feet become pale. The patient then assumes a sitting position with the legs dependent and the times for filling of the foot veins and flushing of the feet measured. The veins should fill within 20 seconds and the feet flush immediately in a warm environment. When these times exceed 30 seconds, the collateral circulation is deemed inadequate, and the patient must be observed frequently for the development of rest pain, ulcers, or gangrene. Venous filling times are of limited value when varicose veins coexist.
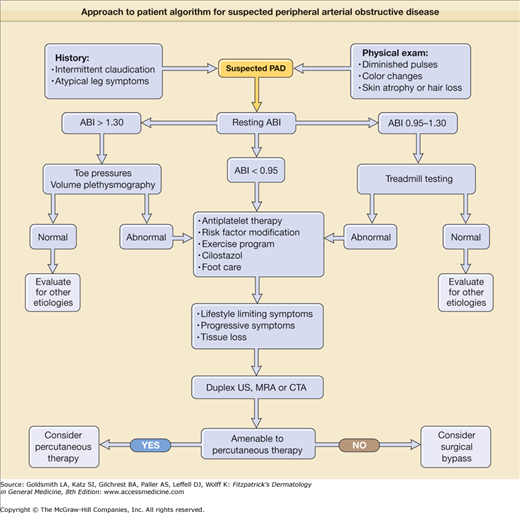
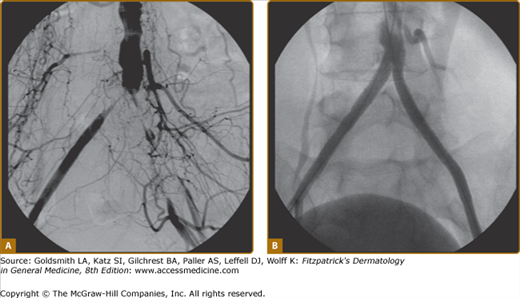
A simple objective test comparing systolic blood pressure in the arms and ankles is sufficient to document the severity of arterial obstruction. The ankle systolic pressure in the supine position should be equal to or greater than the brachial artery systolic pressure. An ankle-to-arm systolic pressure ratio is calculated (ankle-brachial index, or ABI); values greater than 0.9 are generally considered normal, whereas those less than 0.4 are indicative of severe disease (see Chapter 174 for details of ABI measurements). This information is of prognostic use for determination of wound healing or the need for revascularization. One important caveat to note is that occasional patients with heavily calcified or “noncompressible” vessels, most commonly diabetics, may have falsely elevated ABIs (greater than 1.3) despite the presence of significant PAD. Under this circumstance, alternate tests, including toe pressures (as smaller vessels are rarely affected) or segmental volume plethysmography, are helpful. Duplex ultrasound scanning can be time-consuming, but further helps to define physiology and anatomic extent of disease. Magnetic resonance angiography (MRA) and computed tomographic angiography are competitive technologies used to demonstrate precise anatomic location and extent of disease in the arteries but supply limited hemodynamic information. MRA is often preferred because of lack of ionizing radiation or need for iodine-based contrast dye, but may not be tolerated by claustrophobic individuals. Conventional catheter-based arteriography is usually reserved for definitive evaluation in patients about to undergo vascular surgery or as a necessary component of percutaneous angioplasty or stenting procedures (Fig. 173-4).
The diagnosis of PAD can usually be made by the typical history of intermittent claudication and palpation for diminished or absent pulses in the limbs. The ABI is usually diminished, although occasional patients may have normal values at rest. When the diagnosis remains in doubt, a helpful provocative maneuver to increase blood flow is to exercise the patient. Postexercise blood flow may be limited by the arterial obstructive disease, and repeat ABI will then be diminished. The differential diagnosis of PAD includes both local factors and systemic disorders (Box 173-2). Arterial obstructive disease is just one of several potential etiologies for the development of a foot ulcer (Box 173-3). In some patients with diabetes mellitus or other disease resulting in neuropathy, ulcers may develop on the heel, toes, or anterior calf in the presence of normal pulses. These painless (neurotrophic) ulcers are due to repetitive trauma not noticed by the patient because of the peripheral neuropathy. Surrounding callus formation is a typical finding.
LOCAL FACTORS
SYSTEMIC DISEASE
|
Thromboangiitis obliterans also causes intermittent claudication, ulcers, and gangrene. It occurs in young smokers (onset of symptoms before the age of 45 years) and is often associated with superficial thrombophlebitis and vasospasm. In contrast to PAD, it affects medium and smaller arteries and the upper extremities more commonly.
Occlusive vascular disease confined to focal anatomic locations in young patients with minimal traditional risk factors for atherosclerosis should raise suspicion for alternative etiologies. For example, popliteal artery occlusion may occur secondary to entrapment by calf muscles or cystic adventitial disease. In the former, an abnormal anatomical insertion of the medial gastrocnemius muscle head causes compression of the popliteal artery, with the tibial pulses disappearing on plantar (or dorsi) flexion of the foot and full extension of the knee. Pain is aggravated with walking but less with running because knee extension is not as severe with running. In the latter condition, adventitial cysts of unclear etiology compress the vessel lumen, most commonly in the popliteal artery (85%) or rarely, in the external iliac or femoral arteries, presenting as intermittent claudication. Surgical excision of the cysts usually alleviates symptoms although, in the event of complete vessel occlusion, an interposition graft may be necessary.
Neurogenic claudication (pseudoclaudication) is often a difficult diagnosis to distinguish and is due to compression or intermittent ischemia of the lower spinal cord or cauda equina with exercise. Etiologic factors are prolapsed intervertebral discs, congenital stenosis, or hypertrophic bony ridging of the spinal canal. In contrast to PAD, leg pain may occur in the erect position without exercise and be affected by changes in posture, neurologic signs may be present before or after exercise, and peripheral pulses are normal. The pain is frequently relieved by leaning forward against a solid surface or by sitting. Dilemmas in diagnosis and management can occur when both conditions coexist. MRI or computed tomography scan of the spine is used to confirm the diagnosis.
The major direct complications of PAD relate to limb loss from progressive severe ischemia or superimposed infection. In patients with rest pain or tissue loss, the risk of infection is high and wound healing slow or absent. Under these circumstances, the need for revascularization is more urgent to avoid the need for amputation. Superimposed infection needs to be aggressively treated with antibiotics, wound débridement, and local foot care and, if rapidly progressive, presents a medical emergency.
The most feared consequence of PAD is a severe limb-threatening ischemia leading to amputation. Fortunately, the peripheral vascular outcomes in patients with intermittent claudication as a consequence of PAD tend to be relatively benign, with studies showing that 60%–90% of such patients remained stable over a period of 5–9 years.3 In a large prospective study of 1,440 patients with intermittent claudication, only 12.2% patients were reported to require amputation during a follow-up period of 10 years. Occasional patients show spontaneous improvement in symptoms, most likely secondary to enhanced collateral blood flow, although plaque regression is possible. However, patients with diabetes mellitus tend to have progressive disease, and their amputation rate is fourfold greater than for patients without diabetes. In patients with diabetic neuropathy, trauma to the limbs must be avoided, and special shoes may be required. Ongoing smokers also have greater amputation and vascular graft occlusion rates than nonsmokers.
In contrast with the limb-related outcomes, because patients with PAD often have advanced atherosclerosis present in multiple vascular beds, the risk of associated cardiovascular morbidity and mortality is high.1,2 The 5-year mortality in patients with intermittent claudication is 30%, with death largely attributed to cardiovascular causes. In addition, another 20% of patients will incur a nonfatal myocardial infarction or stroke. The association between PAD and cardiovascular morbidity and mortality has been shown to be independent of prior history of cardiovascular disease and independent of known cardiovascular risk factors. Studies have also shown that more severe PAD, as indicated by a lower ABI value, is associated with greater risk of cardiovascular mortality than mild PAD, as evidenced by a higher ABI value. It should be emphasized that PAD of any severity (as documented by an abnormal ABI) may be used to identify the individuals with an increased risk of cardiovascular events and mortality.4
The goal of medical management should include measures to halt the progression of the disease in addition to alleviating the symptoms. The measures to halt the progression of disease include cessation of smoking and optimization of risk factors, such as diabetes mellitus, hypertension, and hyperlipidemia. For patients with symptoms of intermittent claudication, an exercise program is often the treatment of choice (Box 173-4).2 Patients are instructed to exercise to the threshold of tolerable pain, briefly rest, and then exercise again for a total duration of 30–60 minutes a day in excess of their normal activity. The exercise period must be performed in one session, with walking being the preferred modality. Drawing on data from several studies, approximately 80% of patients may be expected to show significant improvements in exercise tolerance through these techniques. While the exact mechanism for improvement in walking distance with exercise remains unknown, regular exercise is thought to condition the muscles to work more efficiently (more extraction of blood) and increase collateral vessel formation. The magnitude of benefit associated with exercise programs for claudication appears to be greater than that reported for clinical trials of pharmacologic therapy. Patients should be further instructed to keep their feet warm, clean, and dry; and extremes of temperature should be avoided because ischemic tissue is more susceptible to burning and to frostbite than normal tissue. Cuts or severe bruises of the limbs or feet should be treated immediately. Conventional vasodilators appear to have no value in treatment.
Symptom Relief | Risk Reduction | |
|---|---|---|
First line | Exercise regimen Cilostazol (Pletal) Pentoxifylline (Trental) | Smoking cessation Statin therapy (target low-density lipoprotein <100 mg/dL) Aspirin Glycemic control Antihypertensive therapy Other lipid therapy |
Second line | Percutaneous revascularization (angioplasty, stent, atherectomy, cryotherapy) Surgical revascularization (endarterectomy or bypass) | Clopidogrel (Plavix) |
Two agents have been approved for the indication of intermittent claudication in the United States. Cilostazol, a phosphodiesterase inhibitor with antiplatelet and vasodilatory properties, has been shown in several studies to have consistent benefits on treadmill walking distance and quality of life.5 However, its benefits are balanced by high frequency of side effects, principally gastrointestinal symptoms or headaches. Furthermore, it is contraindicated in patients with congestive heart failure. Pentoxifylline affects red cell deformability and blood viscosity but has been shown to be relatively ineffective in the treatment of claudication2. Other agents, such as propionyl-l-carnitine an agent with metabolic effects, are under study in the United States for this indication.
Endovascular intervention with angioplasty or stenting is highly effective for aortoiliac disease and is often indicated for moderate, or lifestyle-limiting claudication. Angioplasty or stenting of the superficial femoral artery is technically feasible but limited in applicability by high restenosis rates, particularly in the setting of long occlusions, a common scenario in this location. Despite this limitation, it is a valid therapeutic option for patients with focal disease, severe claudication, or tissue loss. Endovascular management of infrapopliteal (below the knee) disease is associated with extremely high restenosis rates, and is usually a temporizing measure to allow increased blood flow for wound healing. Surgical bypass techniques are effective but are generally high-risk procedures in a population with frequent comorbidities and are usually reserved for patients with severe intermittent claudication or rest pain, or to allow healing of ulcers and gangrene. Sympathectomy is not of value for intermittent claudication, but has been used in the past to allow small ulcers or areas of gangrene to heal. In patients with rest pain, ulcers, or gangrene who are technically unrevascularizable or very high risk for surgery, treatment options are limited. A period of rest with legs dependent may improve some patients, but amputation is a frequent outcome. Parenterally administered prostacyclin or prostaglandin E1 despite some favorable reports of success, has not yet been thoroughly evaluated and is an expensive mode of therapy.
Dry gangrene of the digits or lower limbs should be allowed to spontaneously demarcate. Soaking or ointments are unnecessary. The edges of the gangrenous areas should be kept open if possible and observed frequently for infection. Pain medication is usually necessary for 2–3 months with digital gangrene. A conservative approach and patience will save many digits and extremities. Infected (wet) gangrenous areas must be actively débrided and appropriate antibiotics administered. Amputations may be necessary.
The grim statistics regarding cardiovascular outcomes in these patients emphasize the need for aggressive secondary prevention by modifying appropriate cardiovascular risk factors when possible. Tobacco smoking is absolutely contraindicated. Continued smoking has been identified as the most consistent adverse risk factor associated with the progression of the disease. Smoking even one or two cigarettes a day can interfere with PAD treatments. Smoking is also associated with significantly higher rates of amputation compared with nonsmokers. The rate of in-hospital amputation was shown to be 23% in smokers and 10% in nonsmokers. On the other hand, quitting smoking has been shown to slow the progress of PAD and also to reduce the rates of amputation required. Hypertension and diabetes mellitus should be controlled, and hyperlipidemias should be treated with a target low-density lipoprotein <100 mg/dL (or <70 mg/dL with uncontrolled or multiple risk factors) as per recent guidelines for secondary prevention. Evidence is accumulating that aggressive lipid management will prevent progression of PAD, in addition to prevention of myocardial infarction and stroke. Similarly, antiplatelet use with aspirin is used for prevention of cardiovascular events but some controversy of effectiveness in diabetics with PAD. The use of thienopyridines such as clopidogrel (Plavix) may be of additional benefit, but may be associated with higher bleeding rates (particularly with combined therapy) and expense. Use of antiplatelet therapy is a must for those with PAD but should be individualized.
Atheromatous Embolism
|
The epidemiology of atheromatous embolism is poorly defined due to underreporting and difficulties establishing a clinical diagnosis.6 Routine autopsy series of the adult population suggest an incidence of atheromatous embolism of between 0.15% and ∼4%. The incidence increases dramatically in the presence of severe atherosclerotic disease. Atheromatous embolism has been found in more than 20% of deaths following cardiac surgery or angiography. However, it should be emphasized that the rate of clinically detected atheromatous embolism appears to be reasonably low (less than 1%). The atheromatous embolism appears to be more common in males than in females with a reported male-to-female ratio of approximately 3.4:1. It is strongly associated with older age, with the mean age reported to range from 66 to 72 years.
The pathogenesis of atheromatous embolism involves the occlusion of small arteries and arterioles (of 50–900 μm in diameter) by atheromatous debris (or so-called cholesterol crystals) that are dislodged from a proximal atherosclerotic plaque, with this material inciting an inflammatory cascade characterized by leukocyte infiltration followed by monocytes. This inflammatory process leads to further occlusion with thrombus formation, endothelial cell proliferation, and intimal fibrosis, which may result in ischemia, infarction, and necrosis. The clinical picture is characterized by impaired perfusion of the skin and muscle due to small vessel occlusion, although nearly any organ of the body may be involved.
The major risk factor for the development of atheromatous embolism is atherosclerotic disease of the thoracic or abdominal aorta. The risk is higher with more extensive atheroma burden (defined by increased thickness above 4 mm) or unfavorable plaque features such as protruding mobile plaque.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree