Fig. 33.1
(a) Pre-procedure. (b) Seven days postoperative radiofrequency in lower eyelid. (c) Thirty days postoperative radiofrequency plus treatment with hyaluronic acid dermal filler
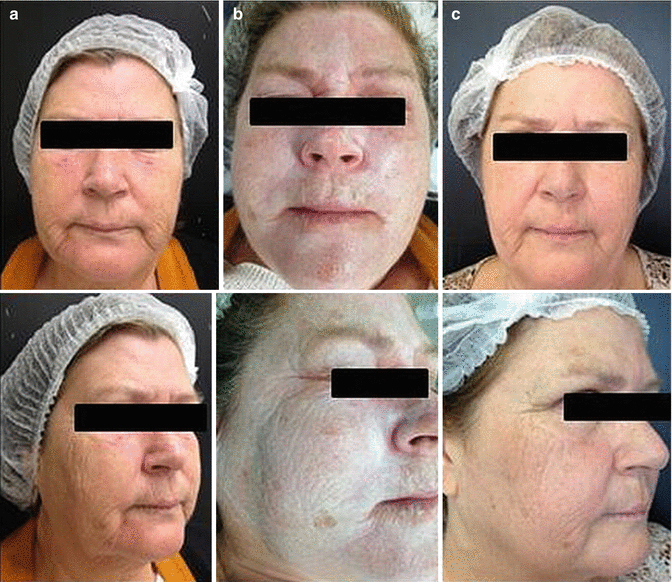
Fig. 33.2
(a) Pre-procedure. (b) Immediately postoperative Jessner’s solution + ATA 25 % peeling. (c) Thirty days postoperative Jessner’s solution + ATA 25 % peeling
Phenol: single agent capable of inducing a deep peel. It may be applied all over the face and is recommended for advanced photo-aging, as well as for the elderly and people with skin types I to III.
It is normally used for localised treatment of perioral and periocular regions. Some complications may include hypopigmentation, skin atrophy, cardiac arrhythmias, renal and laryngeal oedema, exacerbation of concomitant disease and toxic shock syndrome. Therefore, before exposing a patient to such a procedure, laboratory tests (general, liver function, kidney function), in association with heart evaluation, preferably through ECG, are mandatory. During the procedure, patients’ renal and heart functions should be monitored [13–15].
Antimicrobial prophylaxis is recommended to prevent viral and bacterial infections.
Results are excellent and long lasting.
33.4.1.2 Skin Preparation Before Peels
To obtain better clinical response, at least a 4-week regular and continuous preparation is necessary prior to the performance of chemical peels.
33.4.1.3 Application Techniques
Cleaning the skin is essential before the agent of choice may be applied, in order to remove any oiliness that could hinder the absorption of the product [15].
Acetone is still the most used product, which may also be used in an alcohol-based solution, in equal parts.
The chemical agent may be applied with gauze, cotton swabs, brushes and even gloved hands.
33.4.1.4 Contraindications
Inadequate photoprotection
Pregnancy
Stress and neurotic excoriations
Use of oral isotretinoin for less than 6 months (medium and deep peel)
Impaired healing or formation of keloids
History of postinflammatory hyperpigmentation
Difficulty understanding
Unrealistic expectations [14]
33.4.1.5 Major Complications
Medium peels are procedures that require care in the postoperative period. In the first few hours, a rash may appear and also be associated with an oedema during the first days following the procedure, presenting an appearance of “sunburned” skin. In these cases, the use of cold compresses and topical nonsteroidal anti-inflammatory drugs is recommended. The patient should be advised on the scaling, hygiene and use of moisturisers. Uneven pigmentation, prolonged erythema, changes in healing and scarring are possible complications [15].
Medium or deep peels performed carelessly or too intensively may generate unsightly and disfiguring scars. Experience and prudence are required to perform these procedures [13].
The most used type of deep peel is phenol, a substance that has cardiotoxic, hepatotoxic and nephrotoxic effects. There are reports of acute renal dysfunction or exacerbation of chronic renal failure after the performance of phenol peels, as well as the emergence of cardiac arrhythmias or worsening of any pre-existing arrhythmia. Intravenous hydration should be performed both during and after the procedure, in order to prevent renal toxicity.
The use of derivatives of petrolatum helps to maintain hydration and prevent crusting. Reepithelialisation begins after 3 days and is complete in about 1–2 weeks. In some cases erythema can persist for 3 months [13].
33.4.1.6 Post-peeling Guidelines
Keep photoprotection.
Proper hygiene.
After medium and deep peels: avoid intense exercise and sweating; never handle scales or crusts. Patients should not engage in intense activities for 1 week.
Apply topical corticosteroids in case of pruritus, eczematous lesions or prolonged erythema.
Periodic revaluations related to each procedure.
33.4.2 Botulinum Toxin
In dermatology, the main indications for botulinum toxin are treatment of dynamic wrinkles of the face [18] and neck, lifting the tip of the nose, correcting gummy smile and treatment of axillary, plantar and palmar hyperhidrosis. The use of the toxin can be isolated or combined with other aesthetic treatments aimed to prevent facial aging, such as peels, fillers, lasers and others.
Currently, the toxin is a well-known substance, and there is no doubt of its safety and efficacy. Some issues such as dilution, diffusion, duration and effects still remain moot. Botulinum toxin is a neurotoxin produced by a Gram-positive anaerobic bacterium, Clostridium botulinum [12]. The toxin molecule consists of a single polypeptide chain having a heavy chain (110 kD) that has affinity with the endplate responsible for the internalisation of the toxin, while the light chain (50 kD) blocks the release of acetylcholine-dependent calcium [12].
The action of botulinum toxin occurs selectively in cholinergic motor nerve endings, inhibiting the release of the neurotransmitter acetylcholine in the presynapse of the neuromuscular junction, causing a dose-dependent reduction of muscle contraction which results in a flaccid paralysis. The effect starts within 48–72 h or a maximum of 1–2 weeks and may last for 4–6 months [13].
Seven different types of toxins are produced: A, B, C1, C2, E, F and G. The different types are classified according to their cellular target, potency and duration of action, and only serotypes A and B are commercially available.
33.4.2.1 Presentation
Four types of botulinum toxin are available on the market. Botox® was the pioneer product on the market and offers a wide variety of indications, both cosmetic and therapeutic, and is available in 100U. Dysport®, a product already approved for use in 65 countries, differs from Botox® in the process of purification of the toxin. While Botox® is purified by repeated precipitation and redissolution, Dysport® is purified by a column separation method, which can lead to certain changes in the multiprotein complex formed around the toxin. Despite these constitutional differences, clinical applications and efficacy are similar. Xeomin® is the only BTX-A which does not have the multiprotein complex in its structure, bearing no differences in relation to Botox® as to strength, safety and efficacy. Prosigne® is a BTX-A of Chinese origin, with efficacy, safety and tolerability similar to Botox® for the treatment of cervical dystonia [18].
33.4.2.2 Indications
Botulinum toxin carries a lock endplate and is therefore indicated for use in the muscle plane and for the treatment of dynamic wrinkles. The classic applications of the toxin include forehead, glabellar and lateral orbital wrinkles [18]. Among the nonclassical indications for the application of toxin is the treatment of perioral, nasal, chin, cheekbones, neck and presternal wrinkles and lines.
33.4.2.3 Contraindications
Contraindications of use include [18]:
Pregnancy and breastfeeding
Presence of infection around the injection area
Neuromuscular diseases, such as myasthenia gravis, Bell’s palsy and Eaton Lambert syndrome
Hypersensitivity to the components of the formula – botulinum toxin and human albumin
Concomitant use of antibiotic amino glycosides, polymyxin, tetracycline, penicillamine, cyclosporine, calcium channel antagonists and local anaesthetics
Immunosuppression
Collagen diseases
Diabetes
Alcoholism
Coagulation disorders or treatment with anticoagulants
Special attention should be given to patients’ expectations. Unrealistic expectations about the outcome of the procedure, as well as marked skin aging with excessive sagging, may be considered certain contraindications of the procedure.
Every patient should be examined because the anatomy of the facial region may vary considerably.
33.4.2.4 Applications of Botulinum Toxin [18]
Upper third of the face:
Glabella: wrinkles of the glabellar area are formed by the action of the procerus and corrugator muscles. To evaluate this area, ask the patient to pull the eyebrows down and closer together. The product is injected in two to three points in each corrugator and one to two points in the procerus.
Wrinkles in the lateral area of the eyes: wrinkles in the lateral area of the eyes are formed by contraction of the orbicularis oculi muscle. Three to four lateral points can be injected in the indicated area.
Forehead: wrinkles on the forehead are formed through contraction of the frontal muscle. The patient is asked to raise the eyebrows, i.e. to make a face of surprise so that this area may be evaluated. The product may be injected in several equidistant points in this area.
Middle third of the face:
Wrinkles at the top and side areas of the nose: these wrinkles are caused by contraction of nasal muscles and the medial portion of the orbicularis. These wrinkles can be corrected by applying 1 to 2U of toxin above the nasofacial groove. Intradermal application is recommended, with the needle directed from the side to the upper part of the nose.
Lower third of the face:
Diminishment of chin skin’s “orange peel” appearance: the muscles connected to chin wrinkles are the depressors of the lower lip, the depressors of the angles of the mouth and the mentalis muscle. Appearance improvement in the chin area can be achieved by injecting 3–5 U in the midline of the chin, near the edge of the mandible.
Correction of depression of labial commissures: the product is injected in the depressor muscle of the angle of mouth, at a point in the lower extension of the nasolabial folds, near the mandibular arch.
Perioral line wrinkles: these wrinkles can be treated with injections in the midline of the oral orbicularis muscle, near the vermilion border. The injection points should be parallel to the wrinkles, one in each quadrant. This procedure should be performed carefully, always based on small doses, given the risk of paresis and lip incompetence.
Nasolabial or nasogenian folds: these folds can be reduced by injection of the product in the levator muscle of the upper lip. The product should be injected in points parallel to the folds.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





