I. EXTERNAL EAR ANATOMY AND DEVELOPMENT
A. Embryology of the External Ear
1. Auricle
a. Arises from six condensations of mesoderm known as the Hillocks of His during the sixth week of gestation
b. During the eighth week of gestation, the hillocks fuse, forming the rudimentary auricle
c. *Three anterior hillocks derive from the first branchial arch and give rise to the tragus, root of the helix, and helix
d. *Three posterior hillocks derive from the second branchial arch and give rise to the antitragus, antihelix, and lobule
e. The auricle forms in the lower neck and migrates cranially during mandibular development. Arrested growth results in low-set ears.
2. External auditory canal
a. Ectodermal cells (first branchial cleft) initially coalesce forming a meatal plug and later degenerate before birth. If degeneration does not occur, then congenital aural atresia or stenosis results
b. The outer one-third is fibrocartilaginous and the inner two-thirds are bony
c. In adults, it is sigmoid-shaped and approximately 25 mm in length and 10 mm in diameter
3. Tympanic membrane
a. Composed of the pars flaccida (small, triangular, flaccid) and pars tensa (large, oval-shaped, tense)
b. Contains an outer epithelial layer (ectoderm), middle fibrous layer (mesoderm), and inner mucosal layer (endoderm)
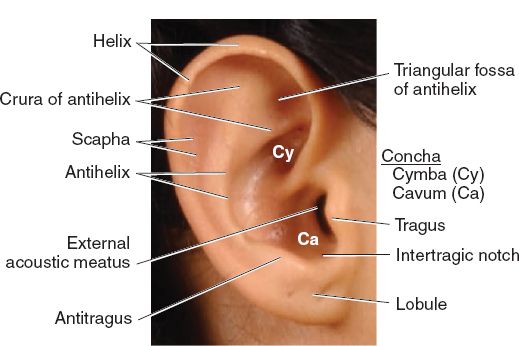
B. Surface topographic landmarks (Fig. 22-1)
C. Vestigial musculature
1. Intrinsic muscles include the helicis major and minor, tragicus, antitragicus, and the transverse and oblique auricular muscles
2. Extrinsic muscles include the anterior, posterior, and superior auricular muscles
D. Vascular system (all branches of the external carotid artery)
1. Posterior auricular artery: *Dominant blood supply to both the anterior (through perforating branches) and posterior surfaces of the ear
2. Superficial temporal artery: Supplies the anterior surface of the ear and forms numerous interconnections with the posterior auricular artery, allowing ear replantation to base solely on either arterial network.
3. Occipital artery: Supplies the posterior surface of the ear (minor contributor) and the retroauricular skin
E. Venous and lymphatic system
1. Venous drainage follows the feeding arteries and empties into the retromandibular vein (external jugular system)
2. Sometimes the occipital vein drains into the internal jugular system
3. Lymphatic drainage parallels embryologic development
a. First branchial arch: Preauricular nodal basin
b. Second branchial arch: Retro- or infra-auricular nodal basins
______________
*Denotes common in-service examination topics
Figure 22-1. Surface anatomy of the ear. (From Moore KL, Dalley AF, Agur AM, eds. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
F. Sensory innervation
1. Great auricular nerve (C2, C3)
a. *Supplies the lower half of both the anterior and posterior surfaces of the ear
b. Arises at Erb’s point which is located 6.5 cm below the tragus along the posterior border of the sternocleidomastoid muscle, and runs parallel to the external jugular vein
2. Auriculotemporal nerve (V3)
a. *Supplies the upper half of the anterior surface of the ear and the anterior aspect of the external auditory canal
b. Ascends with the superficial temporal vessels
3. Lesser occipital nerve (C2)
a. *Supplies the upper half of the posterior surface of the ear
b. Arises above Erb’s point and ascends along the posterior border of the sternocleidomastoid muscle
4. Auricular branch of the vagus nerve (X, Arnold’s nerve)
a. *Supplies the concha and the posterior aspect of the external auditory canal
b. *A ring block will not provide adequate anesthesia to the concha; direct local infiltration is required
5. External auditory canal receives sensory innervation from cranial nerves V, VII, IX, and X
G. Clinical measurements of the ear
1. Approximately 85% of ear growth is achieved by the age of 3
a. Ear width reaches full size by the age of 10, while ear height continues to grow into adulthood
b. *In practical terms, near-adult size is routinely considered between the ages of 6 and 7
2. The ear is located roughly 6 cm, or a single ear-length, posterior to the lateral orbital rim
a. The superior aspect of the helix is at the level of the lateral brow
b. The inferior aspect of the lobule is at the level of the nasal ala
3. Average ear dimensions are 65 mm in height and 35 mm in width (ear width is approximately 50% to 60% of ear height)
4. Relative to the vertical, the long axis is inclined posteriorly 20 degrees
5. On frontal view, the helical rim is slightly lateral to the antihelical fold
6. Auriculocephalic angle: The angle formed between the midpoint of the lateral helix and the mastoid bone (normally between 20 and 30 degrees)
7. Scaphoconchal angle: The angle formed between the scapha and the concha (normally less than 90 degrees)
8. Normal auricular projections include *10 to 12 mm at the upper third, *16 to 18 mm at the middle third, and *20 to 22 mm at the lower third
II. RECONSTRUCTION OF CONGENITAL AURICULAR DEFORMITIES
A. Microtia
1. Epidemiology
a. Overall incidence is approximately 1 in 6,000 births
b. Male-to-female ratio is 2:1
c. *Right/left/bilateral ratio of 5:3:1
d. Most cases are isolated and sporadic; however, possible underlying causes include ischemia (e.g., acute vascular obstruction), drugs (e.g., thalidomide, isotretinoin, retinoic acid), and infection (e.g., rubella)
e. *Syndromes commonly associated with microtia include hemifacial microsomia, Goldenhar syndrome, and Treacher–Collins syndrome
f. Based on embryologic development, the inner ear is often spared, but defects of the external auditory canal and middle ear are common
i. Hearing loss is predominantly due to atresia or stenosis of the external auditory canal, but can result from the absence or structural abnormalities of the ossicular chain
ii. Accordingly, a conductive component (80%) is more prevalent than a sensorineural component (20%)
2. Classification
a. Many different classification systems have been proposed, but they are rarely clinically useful
b. Classification based on the severity of the deformity
i. Grade I: Small auricle with preserved surface topographic landmarks
ii. Grade II: Smaller auricle than grade I with malformed surface topo-graphic landmarks
iii. Grade III: Small sausage-shaped vertical remnant of skin containing a nubbin of hypoplastic cartilage superiorly and a rudimentary lobule inferiorly
iv. Grade IV: Anotia, or total absence of the auricle
3. Timing of Repair
a. *Ear reconstruction is usually delayed until after the ear reaches near-adult size, so if no growth occurs in the reconstructed ear postoperatively, it will still approximate the size of the contralateral normal ear when the patient reaches adulthood
i. General rule of thumb is to wait until the patient requests surgery (i.e., not the parents) because then the patient is aware of his or her deformity and is more likely to comply with the postoperative restrictions
ii. Brent technique: Performed at the age of 6
iii. Nagata technique: Performed at the age of 10. The patient’s chest circumference needs to measure at least 60 cm at the level of the xiphoid process.
b. In the presence of conductive hearing loss, coordination of operative care with an otolaryngologist is paramount
i. In cases of unilateral microtia with normal contralateral hearing, bone-anchored hearing aids (BAHAs) and middle ear reconstruction are not routinely required
ii. *If unilateral conductive hearing loss is present, BAHA placement should be deferred until after ear reconstruction to avoid compromising the vascularity of the skin envelope
4. Surgical management of microtia
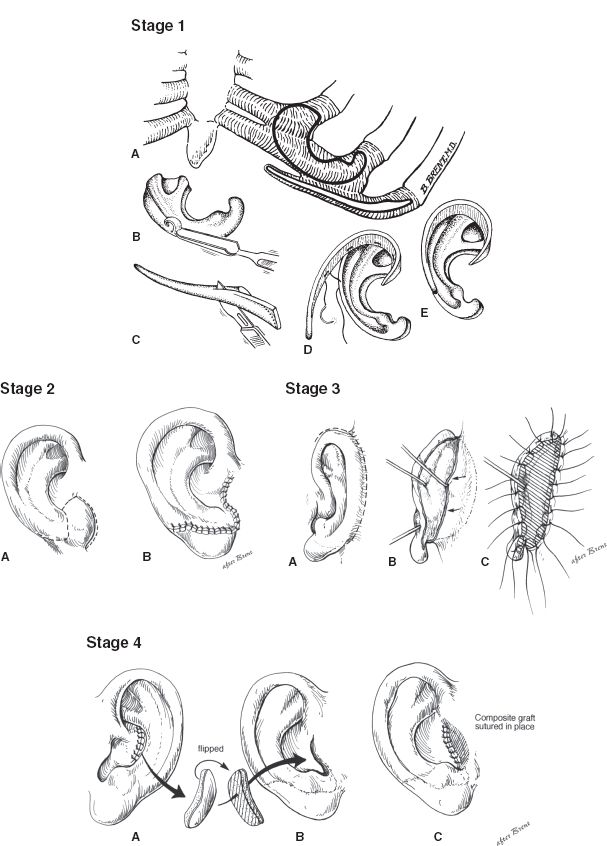
a. Brent technique (four stages) (Fig. 22-2)
Figure 22-2. Brent technique for microtia reconstruction. Stage 1: A-D: The base plate of the framework and the helix and the crura are carved from autologous costal cartilage grafts. The framework is implanted subcutaneously. Stage 2: A-B: The lobule is created by transposing a flap from the microtia remnant or adjacent skin. Stage 3: A-C: The ear is elevated and then a skin graft is placed on its posterior surface to increase lateral projection from the head. Stage 4: A-C: The tragus is reconstructed using a contralateral composite conchal cartilage graft, which is then secured to the previously placed framework.
i. Stage 1: A cartilaginous ear framework is carved from the synchondrosis of the contralateral sixth through eighth ribs, which is then inserted into a subcutaneous pocket beneath the retroauricular skin
ii. Stage 2: Lobular transposition is performed
iii. Stage 3: Elevation of the ear framework, creation of the retroauricular sulcus, and coverage of the posterior reconstructed ear with a full-thickness skin graft
iv. Stage 4: Conchal excavation and tragal reconstruction
b. Nagata technique (two stages)
i. Stage 1: A cartilaginous ear framework is carved from the synchondrosis of the ipsilateral sixth through ninth ribs, which is then inserted into a subcutaneous pocket beneath the retroauricular skin. Lobular transposition and tragal reconstruction are both performed during this initial stage
ii. Stage 2: Elevation of the ear framework, creation of the retroauricular sulcus, and coverage of the posterior reconstructed ear with a tunneled temporoparietal fascial flap (TPFF) and split-thickness skin graft
c. Alloplastic ear framework
i. Options include silicone elastomer (Silastic, Cronin) or porous polyethylene (Medpor, Reinisch)
ii. Major advantage is no donor site morbidity, but higher rates of infection and extrusion
iii. Coverage with a TPFF decreases the complication rate
d. Ear prosthesis
i. Previously considered a poor option because adhesives are required for the prosthesis to “stick” to the patient’s head
ii. With the advent of osseointegrated titanium implants, ear prostheses are now more practical and widely used
iii. *Indications include paucity of local tissue, radiation, burn, trauma, cancer, and in the elderly
iv. In the microtia patient, it is also indicated as a salvage attempt after failed autogenous reconstruction
v. Requires meticulous daily hygiene to prevent the skin-abutment interfaces from becoming inflamed/infected
5. Complications
a. Skin necrosis
i. Tips for minimizing risk include using a closed-suction drainage system, avoid pressure dressings, and do not raise too thin of a flap when creating the subcutaneous pocket
ii. Small areas of partial-thickness necrosis can be managed conservatively with local wound care
iii. Larger areas of full-thickness necrosis require excisional debridement and coverage with a local flap
b. Infection
i. Early recognition and treatment are the mainstay of therapy
ii. *Superficial infections can sometimes be managed nonoperatively with antibiotics alone
iii. *Deep infections (i.e., gross purulence or suppurative chondritis) require irrigation of the subcutaneous pocket, drain placement, and removal of the ear framework
c. Hematoma
i. *Classically presents as sudden onset unilateral ear pain
ii. Treatment is immediate clot evacuation
d. Hypertrophic scarring
i. Commonly occurs at the chest wall donor site
ii. It is important to design incisions that will not interfere with future breast growth and development in female patients
e. Hair growth
i. Commonly occurs in patients with a low temporal hairline
ii. Our preferred treatment is preoperative laser hair ablation
f. Pneumothorax
i. If suspected intraoperatively, air within the pleural cavity can be evacuated using a red rubber Robinson catheter
ii. Rarely requires placement of a thoracostomy tube
iii. Chest plain film is obtained in recovery and on the first postoperative morning
i. Usually more noticeable in thinner patients and when more donor rib cartilage is harvested
ii. Cartilage may regenerate if the perichondrium is left intact
h. Resorption of the cartilage graft
i. Usually due to infection or a tight, restrictive skin envelope
ii. If severe, may require regrafting
iii. Most cartilage grafts retain their size and shape, or grow slightly larger over time
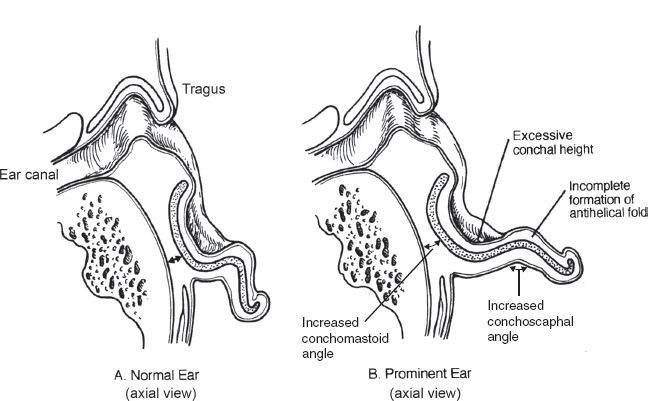
B. Prominent ear (Fig. 22-3)
1. Underdeveloped antihelical fold
a. Most common cause of the prominent ear
b. Defined as a scaphoconchal angle more than 90 degrees
c. Typically results in prominence of the upper third of the ear
2. Conchal hypertrophy
a. Defined as excess conchal cartilage (more than 1.5 cm deep)
b. Typically results in prominence of the middle third of the ear
3. Protruding lobule
a. Least common cause of the prominent ear
b. Typically results in prominence of the lower third of the ear
4. Surgical management of the prominent ear
a. *In newborns less than 6 weeks of age, ear molding can be performed to help reshape the deformed ear
i. *Circulating maternal estrogens lend malleability to the ear cartilage, which persists longer in breastfeeding mothers
ii. Soft putty is shaped into a custom mold that can be adjusted as the newborn grows (worn for several weeks to months)
b. From our experience, the optimal timing for otoplasty is when the patient can actively participate in the decision for surgery, which is usually around 6 to 7 years of age (similar to microtia patients)
c. The type of otoplasty performed will depend on the anatomic abnormality present, and often more than one technique is required
d. Cartilage-scoring techniques
i. Based on Gibson and Davis’ Law, which states that cartilage bends away from the scored surface due to the release of intrinsic stresses
Figure 22-3. Comparison of normal and prominent ear anatomy.
ii. Stenström: Anterior scoring via an anterior approach
iii. Chongchet: Anterior scoring via a posterior approach
e. Cartilage-suturing techniques
i. *Conchoscaphal (Mustardé) sutures: Recreate the antihelical fold using permanent mattress sutures, thereby reducing upper-third prominence
ii. *Conchomastoid (Furnas) sutures: Reduce the auriculocephalic angle, and consequently middle-third prominence, using permanent mattress sutures
f. Cartilage-incising or -excising techniques
i. A powerful tool in patients with stiffer ear cartilage or those with very severe deformities, but the major drawbacks are palpable step-offs and an overly chiseled appearance
ii. Converse–Wood–Smith: Recreates the antihelical fold by first incising the cartilage and then placing permanent mattress sutures
iii. Luckett: Similar to Converse–Wood–Smith, except that a crescent-shaped piece of skin and cartilage is excised
g. Lobule repositioning
i. Correction of upper- and middle-third prominences may reveal or accentuate prominence in the lower third of the ear
ii. Webster: Repositions the helical tail next to the concha, but is often ineffective because the helical tail does not extend into the lobule
iii. Other techniques involve either fusiform, wedge, or fishtail excisions on the posterior surface of the lobule, and then suturing the fibrofatty tissue of the lobule to either the concha or mastoid periosteum
4. Complications
a. Recurrence
i. More common in patients with stiffer ear cartilage
ii. *Early recurrence is most likely due to suture pull-through or suture breakage
b. Asymmetry and contour irregularity
i. More common with cartilage-incising or -excising techniques
ii. Telephone deformity: Relative upper- and lower-third prominences of the ear caused by either overcorrection of the middle third or undercorrection of the upper and lower thirds
iii. Hidden helix: The helix is unable to be seen on frontal view due to overcorrection of the upper and middle thirds
c. Infection
i. Superficial infections can sometimes be managed nonoperatively with antibiotics alone
ii. If not recognized early, suppurative chondritis may develop leading to cartilage loss and residual deformity
iii. Spit sutures can be irritating and should be removed
d. Hematoma
i. *Classically presents as sudden onset unilateral ear pain
ii. Treatment is immediate clot evacuation
e. Keloids
i. More common in dark-skinned patients
ii. Treat initially with pressure earrings and steroid injections
iii. In severe cases, surgical excision and postoperative radiotherapy may be required
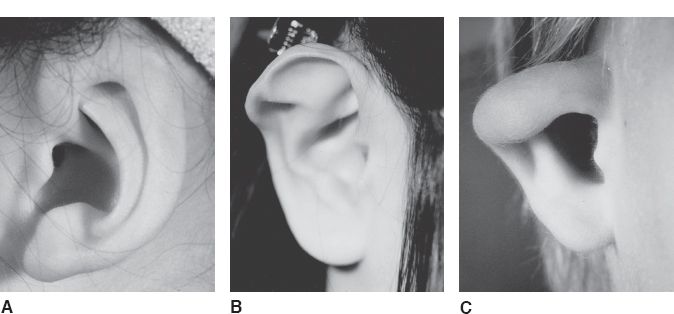
C. Cryptotia (Fig. 22-4A)
1. *Defined as adherence of the superior helix to the temporal skin with absence of the superior auriculocephalic sulcus
2. Surgical correction involves release of the superior helix from the temporal skin and creation of a new superior auriculocephalic sulcus using skin grafts or local flaps
Figure 22-4. Congenital ear deformities. A: Cryptotia. B: Stahl’s ear. C: Constricted ear.
D. Stahl’s ear (Fig. 22-4B)
1. *Defined as an abnormal third crus that arises from the antihelix and extends horizontally to the helical rim
2. The superior crus is often hypoplastic or absent
3. The scapha is malformed, while the concha is normal
4. Surgical correction involves wedge excision of the third crus with helical advancement
E. Constricted ear (Fig. 22-4C)
1. Also referred to as a cup ear or lop ear deformity
2. *Defined as an inadequate circumference of the helical rim causing the superior helix to fold over the scapha
3. Surgical correction involves detaching the superior helix from the scapha and reattaching it at the proper position and angle
III. RECONSTRUCTION OF ACQUIRED AURICULAR DEFORMITIES
A. Epidemiology
1. Malignancy
a. The external ear is prone to sun exposure and development of cutaneous malignancies (10% of all head and neck skin cancers)
b. Squamous cell carcinoma is the most common and has a higher rate of nodal metastasis compared to other head and neck sites
c. *Chondrodermatitis nodularis chronica helicis
i. Common in elderly men related to trauma from sleeping
ii. Presents as a painful, inflammatory papule on the helix due to cartilage inflammation eroding through the overlying skin
iii. Treatment is excisional biopsy, but the recurrence rate is high, so patients should avoid sleeping on the affected ear
2. Trauma
a. Hematomas are caused by skin shearing from the cartilage
i. *Treatment involves immediate clot evacuation followed by a tie-over-bolster dressing to prevent fluid reaccumulation between the perichondrium and cartilage
ii. If the clot is not evacuated, it will undergo fibrosis and calcification resulting in a cauliflower ear deformity
b. Simple lacerations should be minimally debrided to remove avascular tissue prior to wound closure
i. Small lacerations can be easily closed using a single-layer technique (i.e., skin-only closure)
ii. Large lacerations should be closed using a double-layer technique by first reapproximating the cartilage to reduce tension on the wound edges
c. Avulsions and amputations can be considered for replantation depending on the quality of the avulsed/amputated tissue, mechanism of injury, and the overall clinical status of the patient (i.e., a sharp laceration near the base of the ear has the best chance of survival)
3. Burn and frostbite injuries
a. Burn
i. Initial management includes fluid resuscitation, local wound care, pressure relief, avoidance of pillow friction, and topical application of *mafenide acetate due to its superior cartilage penetration (side effects include pain and hyperchloremic metabolic acidosis due to carbonic anhydrase inhibition).
ii. Allow injured tissues to demarcate (may take days to weeks) prior to definitive reconstruction
iii. Small burns may heal secondarily with dressing changes
iv. Large burns with exposed cartilage require well-vascularized soft tissue coverage, and the choice of donor site will depend on the zone of injury (e.g., if the temporoparietal fascia is injured, then it is not available for use)
v. *Complete auricular loss will require total ear reconstruction in a delayed fashion using either a costal cartilage ear framework with a TPFF or pre-expanded local flap for coverage (if the local tissue is not severely scarred), or an ear prosthesis with osseointegrated titanium implants
b. Frostbite
i. Initial management includes rapid rewarming, *use of nonsteroidal anti-inflammatory agents (reduces thromboxane production), and topical application of mafenide acetate
ii. Allow injured tissues to demarcate (may take weeks to months) prior to definitive reconstruction
B. Partial-thickness defects
1. Presence of perichondrium
a. Small defects can heal secondarily with local wound care
b. Full-thickness skin grafts are recommended for larger defects
2. Absence of perichondrium
a. Most defects require coverage with a local flap
i. Helical rim: Same flaps used for full-thickness defects of the upper and middle thirds
ii. Conchal bowl: Trapdoor flap, postauricular island “revolving door” flap, and bipedicle advancement flap
b. Defects less than 1.5 cm near the helical rim can be converted to a full-thickness defect by wedge or shield excision and closed primarily
3. Involvement of the external auditory canal
a. Stenosis is a common long-term complication
b. Treatment is application of a full-thickness skin graft over an acrylic mold used as a temporary stent
C. Full-thickness defects of the upper third
1. Primary closure
a. Commonly performed after wedge or shield excision of skin cancers involving the helical rim
b. To prevent buckling, a star-shaped resection pattern can be used
c. Limited to defects less than 1.5 cm to avoid a significant size discrepancy with the contralateral side
2. Contralateral chondrocutaneous graft
a. For defects up to 1.5 cm and is usually harvested from the contralateral helical rim or conchal bowl
b. The graft should be slightly larger than the defect to account for postoperative contraction
c. *Predictable pattern of color changes during graft take starting with white initially (ischemia), then blue for 24 to 72 hours (venous congestion), and finally pink after 3 to 7 days (neovascularization)
3. Banner flap
a. A local transposition flap that can be based on either the pre- or postauricular skin
b. Performed with or without a cartilage graft; however, if the defect involves more than 25% of the helical rim, then costal cartilage is required for support
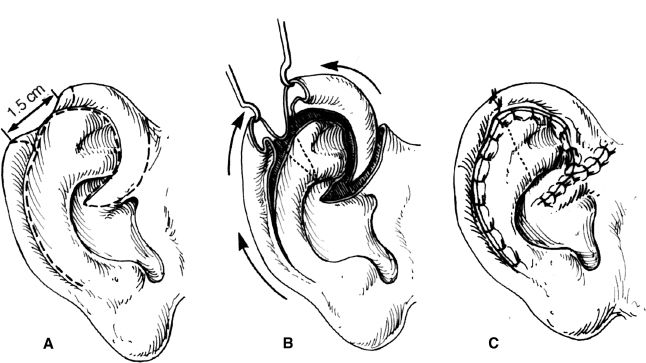
4. Antia–Buch helical advancement (Fig. 22-5)
a. For defects up to 3 cm
b. A transcartilaginous incision is made in the helical sulcus along its entire length from the scapha to the lobule preserving the integrity of the posterior skin
c. The posterior skin is then elevated off the remaining ear cartilage in a supraperichondrial plane until the entire helical rim is mobilized as a chondrocutaneous composite flap (based on the posterior skin)
d. Additional length can be gained by performing a V–Y closure at the root of the helix
5. Chondrocutaneous composite flap
a. For defects involving the superior helical rim, especially when burn patients desire a stump to support their eyeglasses and other local options are not available
b. Anterior skin and cartilage are rotated from the conchal bowl as a composite flap based on either the root of the helix (Davis) or the lateral helical rim (Orticochea)
c. The donor site is skin grafted or left to heal by secondary intention
D. Full-thickness defects of the middle third
1. Similar to full-thickness defects involving the upper third of the ear, reconstructive options include primary closure, contralateral chondrocutaneous graft, Banner flap, and Antia–Buch helical advancement
Figure 22-5. Antia–Buch helical advancement.
a. Based on the retroauricular skin and is performed with or without a cartilage graft in three stages
b. The flap is elevated and tubed with primary closure of the donor site. Three weeks later, one end of the tube is divided and transferred into the defect. Finally, after an additional 3 weeks, the other end of the tube is divided and transferred into the defect.
3. Dieffenbach flap
a. Similar to the tubed bipedicle flap, this flap is based on the retroauricular skin and is also performed in multiple stages
b. A cartilage graft is harvested and placed into the helical rim defect. The anterior surface of the graft is then covered with a postauricular transposition flap. Three weeks later, the flap is divided at its base and inset using the additional length of the flap to cover the posterior surface of the graft.
c. The donor site is skin grafted or closed by local tissue rearrangement
4. Converse “tunnel” technique
a. A prelaminated flap based on the retroauricular skin
b. A cartilage strut is tunneled beneath the retroauricular skin and secured to both ends of the helical rim defect. Three weeks later, the cartilage strut is elevated along with the retroauricular skin still attached.
c. The donor site is skin grafted or closed by local tissue rearrangement
E. Full-thickness defects of the lower third
1. Many techniques have been described to reconstruct the lobule, but the basic premise involves the use of local flaps folded over on themselves
2. The lobule normally does not contain any cartilage; however, contralateral auricular cartilage or nasal septal cartilage can be placed subcutaneously as a graft to provide additional support and contour
3. If the lobule on the contralateral side is enlarged, sometimes it can be surgically reduced to match the reconstructed side
4. For cleft lobules, our preferred technique is wedge excision and primary closure with eversion of the wound edges to prevent postoperative notching
IV. THE AMPUTATED EAR
A. Nonmicrosurgical options
1. Usually involves burying or banking cartilage in the temporal scalp, abdomen, or volar forearm for delayed reconstruction
2. Many techniques have been described, but often the results are inconsistent and the cartilage loses its definition over time becoming flattened and warped
a. Baudet recommended removing only the posterior skin from the amputated segment and fenestrating the cartilage to allow for greater imbibition and neovascularization. The cartilage was covered by a postauricular flap and later divided at 3 months
b. Mladick recommended dermabrading the amputated segment, reattaching it, and then burying it beneath the retroauricular skin. Three weeks later, the reconstructed ear was removed from its “pocket,” and the denuded areas eventually re-epithelialized.
c. Destro and Speranzini recommended removing all the skin from the amputated segment except for the concha and then made small round perforations in the cartilage. The cartilage was covered by a postauricular flap and later divided at 3 months.
d. Park recommended removing all the skin from the amputated segment except for the helical rim. The cartilage was then sandwiched between two flaps: a skin flap anteriorly and a fascial flap posteriorly, both based on the mastoid region. The skin on the helical rim later necrosed, but was salvaged with additional operations.
B. Microvascular replantation
1. *Microvascular replantation provides a more natural-appearing reconstructed ear and is preferred to other forms of delayed reconstruction
2. It is a technically demanding operation due to the small caliber of the vessels, and often venous outflow is a problem
3. Microvascular anastomosis is performed to either the posterior auricular artery or the superficial temporal artery. Both can restore the blood supply to the entire amputated segment given the numerous interconnections that are present between these two arterial networks.
4. If no vein can be found, or the veins are small, have a low threshold for starting leeches postoperatively
PEARLS
1. Patients with oropharyngeal cancer may complain of referred otalgia via Arnold nerve
2. A ring block will not provide adequate anesthesia to the concha; direct local infiltration is required
3. Children with microtia or prominent ears are usually operated on between the ages of 6 and 7 when ear growth is nearly complete
4. For the burned ear, mafenide acetate is preferred due to its superior cartilage penetration, but its application can be painful and beware of hyperchloremic metabolic acidosis from carbonic anhydrase inhibition
5. In newborns less than 6 weeks of age, ear molding can be performed to help reshape many deformed ears due to circulating maternal estrogens lending malleability to the ear cartilage
6. Microvascular replantation may sacrifice the superficial temporal artery, which would impair the use of the temporoparietal fascial flap in the future
7. Costal cartilage is often stiffer and more calcified in adults compared to children, thus the cartilaginous ear framework used for total ear reconstruction in adults is usually carved en bloc.
QUESTIONS YOU WILL BE ASKED
1. What part of the ear is not anesthetized by ring block?
Conchal bowl due to CN X (it requires direct infiltration).
2. What is the most common reason ear replantations fail?
Poor venous outflow.
3. Why is it more difficult to use autogenous cartilage in the elderly for total ear reconstruction?
Calcification of the rib cartilage.
4. What is the most common complication of otoplasty?
Recurrence.
THINGS TO DRAW
Draw the surface anatomy of the ear.
Recommended Readings
Antia NH, Buch VI. Chondrocutaneous advancement flap for the marginal defect of the ear. Plast Reconstr Surg. 1967;39(5):472–477. PMID: 5336914.
Brent B. Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast Reconstr Surg. 1992;90(3):355-374; discussion 375–376. PMID: 1513882.
Brent B. The acquired auricular deformity. A systematic approach to its analysis and reconstruction. Plast Reconstr Surg. 1977;59(4):475–485. PMID: 322165.
Janis JE, Rohrich RJ, Gutowski KA. Otoplasty. Plast Reconstr Surg. 2005;115(4):60e–72e. PMID: 15793433.
Pribaz JJ, Crespo LD, Orgill DP, Pousti TJ, Bartlett RA. Ear replantation without microsurgery. Plast Reconstr Surg. 1997;99(7):1868–1872. PMID: 9180709.
< div class='tao-gold-member'>