23 Complex regional pain syndrome in the upper extremity
Synopsis
 Complex regional pain syndrome (CRPS) is a regional pain syndrome occurring mostly in extremities after trauma that displays chronic regional pain, neurosensory changes, and features of dysautonomia, inflammation, and dystonia.
Complex regional pain syndrome (CRPS) is a regional pain syndrome occurring mostly in extremities after trauma that displays chronic regional pain, neurosensory changes, and features of dysautonomia, inflammation, and dystonia.
 The pain of CRPS may include an element of sympathetically maintained pain (SMP), sympathetically independent pain (SIP), or both.
The pain of CRPS may include an element of sympathetically maintained pain (SMP), sympathetically independent pain (SIP), or both.
 The emerging model for the pathogenesis of CRPS suggests a triad of nerve injury, peripheral sensitization, and central sensitization.
The emerging model for the pathogenesis of CRPS suggests a triad of nerve injury, peripheral sensitization, and central sensitization.
 Contemporary thinking suggests that CRPS I (reflex sympathetic dystrophy: RSD) is frequently related to small-caliber nerve pathology, even when a specific, discrete peripheral nerve injury is not identifiable by traditional means.
Contemporary thinking suggests that CRPS I (reflex sympathetic dystrophy: RSD) is frequently related to small-caliber nerve pathology, even when a specific, discrete peripheral nerve injury is not identifiable by traditional means.
 Diagnosis of the syndrome rests on specific history and physical exam findings, and the lack of other plausible explanations. Various adjunctive testing measures serve mainly to characterize further an individual’s disease and create an individualized approach to therapy.
Diagnosis of the syndrome rests on specific history and physical exam findings, and the lack of other plausible explanations. Various adjunctive testing measures serve mainly to characterize further an individual’s disease and create an individualized approach to therapy.
 Diagnosis of CRPS should always prompt a thorough search for underlying nerve injury, such as nerve compression syndromes or neuromas which can be surgically treated.
Diagnosis of CRPS should always prompt a thorough search for underlying nerve injury, such as nerve compression syndromes or neuromas which can be surgically treated.
 Early recognition and multidisciplinary management, including physical rehabilitation, pharmacologic and procedural pain management, psychological consideration, and peripheral nerve surgery evaluation, are crucial for effective treatment.
Early recognition and multidisciplinary management, including physical rehabilitation, pharmacologic and procedural pain management, psychological consideration, and peripheral nerve surgery evaluation, are crucial for effective treatment.
 When CRPS is due to identifiable nerve injury, treatment of nerve injury should be a primary treatment for the syndrome.
When CRPS is due to identifiable nerve injury, treatment of nerve injury should be a primary treatment for the syndrome.
 A variety of peripheral nerve surgery techniques are indicated for treatment of CRPS, including neurolysis, nerve reconstruction, neuroma resection, and denervation. With proper patient selection, these techniques can provide effective and enduring therapy.
A variety of peripheral nerve surgery techniques are indicated for treatment of CRPS, including neurolysis, nerve reconstruction, neuroma resection, and denervation. With proper patient selection, these techniques can provide effective and enduring therapy.
 Much of the burden of CRPS is due to changes mediated by the central nervous system (CNS). The peripheral nerve surgeon is most effective by preventing peripheral pathology from progressing to central changes.
Much of the burden of CRPS is due to changes mediated by the central nervous system (CNS). The peripheral nerve surgeon is most effective by preventing peripheral pathology from progressing to central changes.
 Understanding the scientific rationale for the new theories behind the development of CRPS provides a logically based directive for peripheral nerve surgeons to become more involved in managing this disorder.
Understanding the scientific rationale for the new theories behind the development of CRPS provides a logically based directive for peripheral nerve surgeons to become more involved in managing this disorder.
Introduction
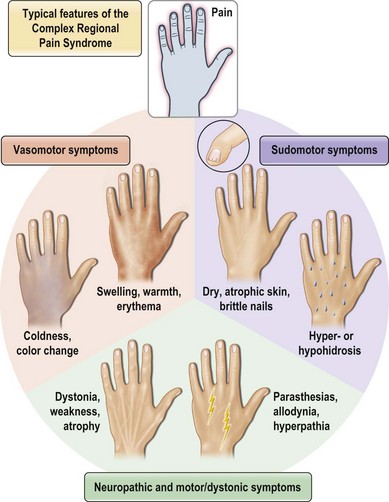
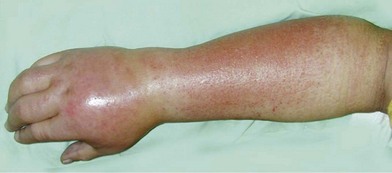
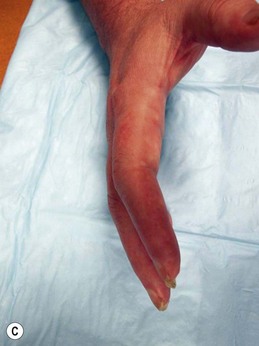
“Complex regional pain syndrome” or CRPS is the moniker given to a disorder that is clinically challenging because it typifies these frustrations. Although the disorder is predominantly one of chronic pain, the full spectrum of its symptomatology and the mystifying sequence of pathophysiology that leads to its development have proven so confusing that even its name and diagnosis are a subject of debate. CRPS type I, formerly known as reflex sympathetic dystrophy (RSD), may develop after any noxious insult, but most commonly after an orthopedic extremity injury, such as a sprain or fracture, and/or related treatments. CRPS type II, formerly known as causalgia, develops specifically after a nerve injury. Both are characterized by persistent pain, but unlike typical postinjury pain or typical neuralgia resulting from a nerve injury, the pain of CRPS may spread to a region beyond the zone of the original insult, may be maintained by adrenergic activity, and may encompass more than the territory of a single nerve. The pain of CRPS is constantly present but is often exacerbated by the presence of tactile allodynia or hyperpathia, to the point where patients may shield or hide the affected body part for fear of it being touched. In addition, the patient develops an array of symptoms, such as edema, osteopenia, vasodysregulation, temperature dysregulation, hyper- or hypohidrosis, or muscle dystonia, that are alarming to patients and physicians because they are more typically associated with autonomic or neurologic dysfunction than with physical injury (see Figure 23.1 for a summary of the complex of symptoms of CRPS). These changes, and possibly the disease process itself, lead many patients to develop comorbid anxiety, depression, and personality changes. Taken together, the pain and associated symptoms have the potential to be debilitating and isolating, and may either spontaneously resolve or progress to a chronic, unremitting form.
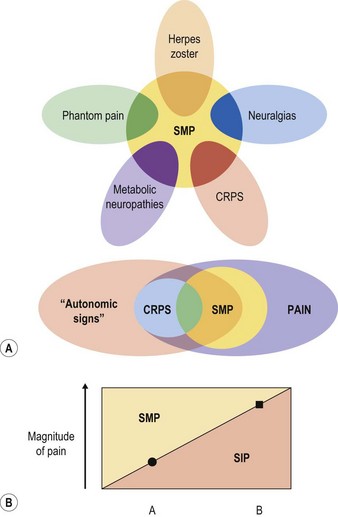
Theories for the underlying cause of the syndrome and their attendant epitaphs have ranged from those of peripheral nervous dysfunction (e.g., “causalgia”), of autonomic hyperactivity (“RSD”), of exaggerated inflammatory response (“Sudeck’s osteodystrophy”), and even psychologic hysteria, among others. Each has found its supporters, but has failed to account for the entire clinical picture. The most contemporary terminology, CRPS, attempts to unify previously separate diagnoses under a less specific but more sensitive umbrella by removing from its name etiologic references (such as “reflex,” “sympathetic,” or “dystrophy”) that are suggestive of underlying models of pathogenesis whose validity have never been borne out. It makes provisions to include disparate features such as sympathetically maintained pain (SMP) (Fig. 23.2) and nerve injury, without requiring their presence for diagnosis. Although the name “CRPS” and its diagnostic criteria were developed at a consensus conference of pain specialists in 1994 (Table 23.1),1 even these are not universally agreed upon,2,3 with many authors preferring the older terms “RSD” and “causalgia.”
Table 23.1 International Association for the Study of Pain criteria for diagnosis of chronic regional pain syndrome (CRPS)
(Reproduced from Stanton-Hicks M, Janig W, Hassenbusch S, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 1995;63:127–133.)
Although multiple theories still coexist, progress is being made in rejecting convenient yet unfounded theories on the etiology of CRPS and developing research-based models that will do a better job both defining the disorder and its risk factors, and yielding more appropriate therapies.4 The most compelling possibility is that all cases of CRPS are caused by some form of peripheral (somatic and/or autonomic) nerve damage. The empiric observation that both forms of CRPS may have identical clinical manifestations but that type II is brought on by a recognizable nerve injury (whereas the symptoms of type I are not as closely linked to a specific cause) is suggestive of a role for nerve injury in both types. As well, there is mounting research evidence to suggest that peripheral nerve damage, whether of large recognizable nerves or of smaller distal fibers, may be the inciting incident in a cascade of events that ultimately leads to the clinical syndrome of CRPS.5–7 This chapter will explain how CRPS is linked to nerve injury, and why this provides a logical directive for the involvement of peripheral nerve surgeons in its treatment.
Pathophysiology
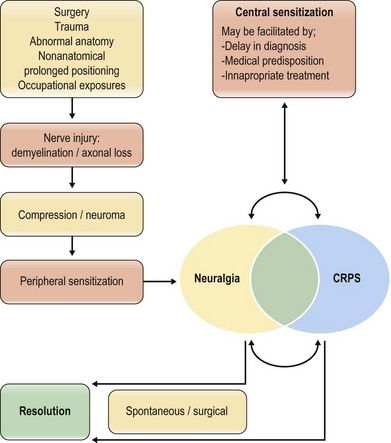
There is no consensus regarding the pathophysiologic mechanisms underlying the development of CRPS. However, most pertinent to peripheral nerve surgeons is the emerging model of CRPS as the ultimate result of untreated nerve injury. We propose the three components of this model: (1) peripheral nerve injury; (2) peripheral sensitization; and (3) central sensitization (Fig. 23.3).
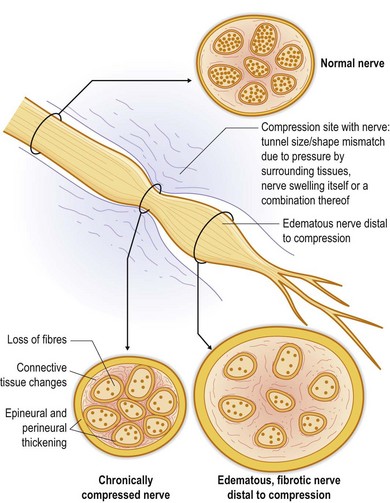
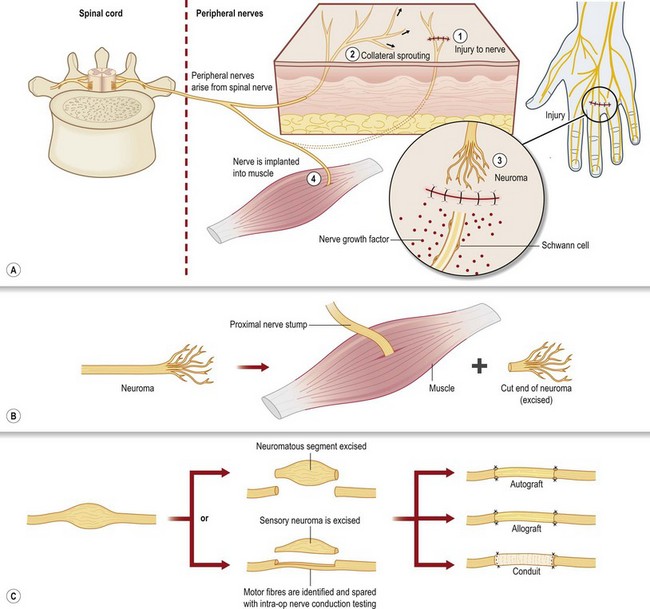
The two most common mechanisms of nerve injury are either from an acute trauma (including surgery), such as by crush, stretch, or transection, or from chronic compression (Fig. 23.4), as is often seen in upper and lower extremity compression syndromes like carpal, cubital, radial, or tarsal tunnel. Nerve injuries may spontaneously recover, or they may result in neuroma, or compression neuropathy – two permanent disruptions in nerve architecture that generate chronic pain. In the case of trauma, the result of nerve injury is often a painful neuroma, where the cut or crushed end of a nerve grows aberrant sprouts in an attempt to regenerate (Fig. 23.5). These sprouts become tangled in surrounding connective tissue, failing to regenerate along their normal course, and causing chronic pain. A variant of this pattern is the neuroma in continuity, where the nerve is not transected, but a crush injury disrupts the internal fascicular organization of the nerve; scar tissue then forms within the nerve, blocking the regeneration of axonal sprouts and creating a neuroma.8,9 The other end point of nerve injury that causes chronic neuropathic pain is compression neuropathy (Fig. 23.4). Compression neuropathy causes nerve ischemia and degeneration by physical pressure on the nerve, which occludes blood flow as well as axonal transport. Compression neuropathy may occur in the setting of a naturally tight anatomic tunnel exacerbated by tissue edema/hypertrophy (such as occurs with overuse syndromes and trauma), or may be the result of nerve entrapment in scar tissue following trauma. Clinically recognizable neuromas or large-nerve compression neuropathies can be the basis for CRPS type II (causalgia), whereas damage to clinically undetectable small distal fibers is likely the basis of CRPS type I (RSD),4,5,10,11 which does not classically include a nerve injury.
Not all nerve injuries progress to CRPS. Some may never be painful, or may be only briefly painful. Some may cause chronic neuropathic pain in the distribution of the injured nerve, but never lead to the regional spread, dysesthesias, trophic changes, and associated dysautonomic symptoms that characterize CRPS. The first step towards the development of CRPS after nerve injury comes with the onset of peripheral sensitization, defined as a progressive increase in the response of peripheral nociceptors to repeated stimuli.12 This increasing sensitivity of nociceptive afferents is brought about both by autosensitization, wherein activation of the receptor itself reduces its own activation threshold, and by heterosensitization, where mediators such as inflammatory cytokines (e.g., prostaglandin E2, bradykinin) and neurotrophic factors increase excitability of the neuronal membrane without activating transducers. In both cases, transduction threshold is reduced, making it easier for an action potential to occur. Noteworthy in the case of CRPS, whose clinical features include regional spread of pain and pain in response to sympathetic stimuli, is the concept that peripheral sensitization does not only affect the injured nerve, which is often distally malfunctioning and/or incapable of producing positive signals. Instead, neighboring nerve endings are sensitized, and there is experimental evidence showing that damage to a nerve causes early and spontaneous ectopic firing of adjacent, uninjured C-fibers. These fibers also develop reduced threshold for ectopic firing; particularly, they become sensitive to alpha-adrenergic activity.13,14
The end result of peripheral sensitization is both an increase in the pain experienced by the patient, as well as an amplification of nociceptive input entering the CNS. Left untreated, and in a chronic form, prolonged and increased nociceptive input to the CNS triggers modulation in central pain pathways, resulting in central sensitization. Central sensitization results in enhanced responsiveness of central pain transmission neurons, which either outlasts the initiating input or requires only a low-level peripheral drive to maintain it.12 Thus, pain which began from a physical insult in the periphery can become centrally generated by the CNS even in the absence of any continued peripheral stimulus. Because the mechanisms underlying this modulation involve changes in receptor (e.g., N-methyl-d-aspartate (NMDA) receptors) expression akin to the long-term potentiation associated with memory in the hippocampus, they are long-lasting and make the disorder difficult to reverse at this point.
Taken together, this combination of nerve injury, peripheral sensitization, and central sensitization can explain many of the perplexing disease features of CRPS. Features of the syndrome that were previously assumed to be the result of abnormal sympathetic outflow can be explained by distal small-fiber loss due to nerve injury. Distal small nerve fibers are mixed in function, consisting of not only sensory (A-delta and C fibers) components, but sympathetic axons, and neuroeffector peptides (e.g., substance P, and calcitonin gene-related peptide) that regulate tissue function. Small fiber loss may cause denervation of distal limb arteriovenous shunts, with loss of resting tone and opening of the shunts.5 This could lead to the asymmetric limb warmth and hyperperemia associated with RSD, while causing paradoxical hypoperfusion of deeper tissues,15 contributing to deep pain. That some CRPS-affected limbs appear blue and cold rather than warm and hyperemic may be explained by eventual supersensitivity of denervated vessels to circulating catecholamines. The sudomotor instability (hypo- or hyperhidrosis) often seen in RSD/CRPS I is also explicable by nerve injury leading to denervation of sweat glands, which are normally innervated by cholinergic sympathetic small fibers. Sweat glands of CRPS I patients have been shown on pathologic examination to be denervated of these cholinergic sympathetic small fibers, but have aberrant ectopic sprouting of adrenergic innervation from nearby nervi vasorum; thus, they may both sweat insufficiently from direct neural stimulation and yet sweat excessively in response to circulating catecholamines.5,16
Remaining disease features are accounted for by peripheral and central sensitization. As previously mentioned, the process of peripheral sensitization can reduce activation threshold as well as induce expression of adrenergic receptors on nociceptive fibers adjacent to an injured nerve13,14; this phenomenon helps to account for both SMP and regional spreading of pain. Spreading of pain and hypersensitivity to regions beyond the injured tissue is also accounted for by the mechanisms of central sensitization, wherein a centrally sensitized neuron can generate pain outside the distribution of the stimulating peripheral input by increasing the excitability of its synapses with multiple other central neurons.12,17 Finally, innappropriate sensitization of both peripheral and central afferents leads to the hyperpathia and allodynia that are characteristic of the pain in CRPS.12,18
Patient presentation
Epidemiology
CRPS occurs with an estimated incidence of 26.2 per 100 000 person-years,19 with a published range of 5.46–26.2 per 100 000 person years.19,20 Published estimates indicate that CRPS I makes up 97% of cases in the general population, with CRPS II being more common in certain subpopulations such as the military as a result of gunshot wounds and shrapnel injuries.19 However, experience in the surgical literature suggests that CRPS II may be underdiagnosed or frequently misdiagnosed as CRPS I.6,7 The syndrome may occur in any age group, although the peak prevalence seems to be in middle age, suggesting that many cases actually resolve and do not progress to a chronic form with disability.19,20 Females are affected at least three times as often as men,19,20 for unclear reasons.
Precipitating events
The most common precipitating events for CRPS I are extremity fractures (e.g., distal radius fractures) and sprains, although surgery, tendon injuries, and crush injuries are also common causes. Seemingly minor or innocuous events, such as phlebotomy, can also be causes, as can nontraumatic insults such as tumors, infarctions, and vasculitis with mononeuropathy multiplex. For known cases of CRPS II, nerve compression syndromes and penetrating injuries (including surgery) are common causes. For both types of CRPS, the upper extremity is more often involved than the lower extremity (60% versus 40%), although as a group, CRPS of the extremities make up the vast majority of cases, with head, trunk, and visceral involvement being possible but rare.21,22 This distribution is in keeping with an underlying etiology of nerve injury, since the axial pattern and proximity to hard tissues of extremity nerves make them more vulnerable to injury than trunk nerves. Upper extremity involvement may be more frequent than lower extremity involvement due to the smaller receptive fields and greater density of innervation in the arm than the leg.
Patient characteristics
The only high-powered study to examine medical history prior to the onset of CRPS found an association between a history of migraines, asthma, neuropathies, osteoporosis, and menstrual cycle-related problems and the later development of CRPS.23 Although the cryptogenic nature of the disease as well as the emotional changes it may produce in patients have caused suspicion for a hysterical or psychological underpinning in CRPS, multiple studies have studied the association of psychological factors with CRPS onset and found no evidence in support of CRPS as a psychologically mediated disease, or of the idea that certain psychological profiles are predisposed to its development.23–25 With this being said, it is important to keep in mind that emotional and psychological changes such as anxiety and depression do develop in chronic CRPS as a consequence of central sensitization, and therefore may be a presenting feature of the disease.
Diagnosis
CRPS is a clinical diagnosis that should be made based on history and physical examination findings. There are several proposed diagnostic criteria, with the 1994 International Association for the Study of Pain (IASP) criteria (Table 23.1) being most frequently cited. Several modifications of these criteria have been proposed in an attempt to improve specificity.26
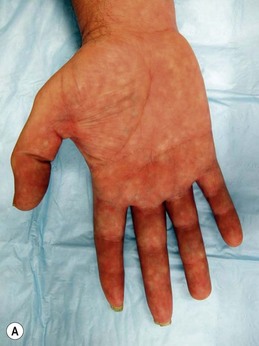
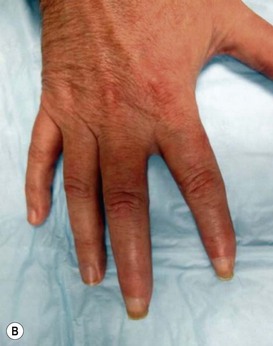
Physical examination should be performed concurrently with the history to augment the identification of nerve injuries. Physical exam begins with a search for outward scars or signs of trauma to the painful region. The presence of any of the typical inflammatory, dysautonomic, or atrophic features of CRPS should be noted (Figs 23.6 and 23.7). The location of the patient’s pain as described in the history should then be mapped on to the surface of the body, and this region should be scrutinized by the examiner for a correlation to specific peripheral nerve distributions. When the distribution of the pain does not match a known peripheral nerve distribution, either multiple overlapping nerve territories may be implicated, or the regional spread of pain via sensitization may have occurred. Once the region of pain has been clarified on physical exam, the presence of a Tinel’s sign in nerves within the region should be tested for by tapping over any known anatomical compression points, obvious scars, or suspected sites of painful neuromas. The presence of a positive Tinel’s sign (a tingling sensation radiating distally from the site of percussion) helps to localize cutaneous neuromas, and signals a greater likelihood of response to surgery for nerve compression injuries, presumably by indicating the presence of surviving neurons distal to the point of injury.
If a cutaneous neuroma is suspected based on history and physical exam, the likelihood that the symptoms of CRPS will resolve with resection and implantation of the neuroma should be assessed using nerve block with local anesthetic. This is performed by infiltrating the region proximal to the suspected neuroma with local anesthetic (the nerve itself is not injected). The response is considered positive when there is a postblock to preblock reduction in pain by greater than or equal to 5 points on a visual analog scale (score of 0–10, with 10 being the worst pain). If a single block fails to produce pain relief, the possibility that a nearby nerve with an overlapping sensory territory is also involved should be investigated by a new block of the suspected adjacent nerve. If the pain remains after sequential block of all possible sensory nerve territories, then there is presumed to be a fixed central mechanism (e.g., dorsal column, thalamus) for the pain and peripheral nerve surgery is unlikely to be of benefit.6
A special note should be made for CRPS that localizes to a particular joint, such as often occurs after joint injury (e.g., wrist fracture) or surgery (commonly knee surgery). After orthopedic evaluation has ruled out biomechanical joint problems, these cases should be treated by the peripheral nerve surgeon as suspected injuries to joint afferent fibers, and the office workup for these patients should be analogous to that for cutaneous neuromas, with response to surgery being predicted by nerve blocks of the joint afferents and surgical therapy, if warranted, being partial joint denervation.6,7,27,28
The final tool in the diagnostic workup used by peripheral nerve surgeons to identify nerve injury and predict response to treatment is electrodiagnostic neurosensory testing (NST). NST in the context of CRPS is indicated mainly for the detection and evaluation of nerve compression syndromes. Although accepted and widespread in their use, traditional electrodiagnostic testing modalities such as electromyography and nerve conduction studies are too painful for CRPS patients, who often suffer from severe hyperpathia and allodynia. Additionally, these tests can have a high false-negative rate for detecting small-fiber disease, which may represent the bulk of CRPS I cases along with CRPS II cases due to neuromas of smaller cutaneous afferents. A less painful and more sensitive test is the quantitation of cutaneous pressure thresholds for one- and two-point discrimination throughout the nerve distributions in question. This can be performed using the Pressure-Specified Sensory Device (PSSD), which has been shown to have improved sensitivity compared to nerve conduction studies29 for the detection of compression neuropathies while being as painless as possible (the device exerts gentle dull pressure without penetrating the skin or giving an electrical stimulus). NST can be used to document the presence of a suspected nerve lesion, to quantify its severity, and to monitor recovery through a period of observation or postoperatively.
Adjunctive diagnostic measures
Tests commonly used to search for evidence of the inflammatory process in CRPS are X-ray and bone scanning. X-ray may reveal the cortical and cancellous osteopenia (Sudeck’s), and subchondral cysts that are commonly associated with the generalized inflammation in early CRPS; if found on X-ray, osteopenia can be treated appropriately (e.g., with bisphosphonates) to reduce morbidity. Of course, occult arthritis or biomechanical joint problems may also contribute to CRPS and should be treated appropriately if detected on X-ray. Radionuclide bone scan with technetium-labeled diphosphonate will also show abnormalities in many CRPS patients. The test is divided into three phases, with diffuse uptake of tracer in the delayed image (phase 3) reportedly correlating well with the diagnosis of CRPS.30–33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree