20 Cold and chemical injury to the upper extremity
Synopsis
Cold injury
 Frostbite should be treated with rapid rewarming in a 40–42°C hydrotherapy tank.
Frostbite should be treated with rapid rewarming in a 40–42°C hydrotherapy tank.
 Frostbite should be treated with oral ibuprofen and topical aloe vera.
Frostbite should be treated with oral ibuprofen and topical aloe vera.
 Common sequelae of frostbite, in general, include residual pain and cold intolerance.
Common sequelae of frostbite, in general, include residual pain and cold intolerance.
 Less common sequelae of frostbite in children include shortened digits and angular deformity due to injury to the growth plate.
Less common sequelae of frostbite in children include shortened digits and angular deformity due to injury to the growth plate.
Extravasation injury
 Extravasation involving small to intermediate amounts of agent may be managed with elevation and observation versus liposuction or saline flushout performed within 24 h of extravasation.
Extravasation involving small to intermediate amounts of agent may be managed with elevation and observation versus liposuction or saline flushout performed within 24 h of extravasation.
 Significant extravasation carries a high risk of skin ulceration and should be addressed with early surgical intervention.
Significant extravasation carries a high risk of skin ulceration and should be addressed with early surgical intervention.
 Doxorubicin extravasation can be managed with IV administration of dexrazoxane within 6 h of extravasation.
Doxorubicin extravasation can be managed with IV administration of dexrazoxane within 6 h of extravasation.
 If compartment syndrome develops, prompt fasciotomy is indicated.
If compartment syndrome develops, prompt fasciotomy is indicated.
Cold injury
Frostbite is the most common local cold injury, although cold exposure does not always result in tissue freezing. Rather, there is a continuum that ranges from minimal skin chilling to tissue freezing with ice crystal formation.1 The two key factors that determine the resultant type of cold injury are the rate of cooling and the presence or absence of ice crystal formation in the tissues.2 Frostnip occurs due to rapid temperature drops without formation of ice crystals. It causes skin pallor and numbness but is completely reversible.3 Flash freezing occurs due to contact with cold metal or volatile liquids that results in rapid cooling with formation of ice crystals. Frostbite occurs when the tissue is cooled slowly with formation of ice crystals when the tissue freezes at –2°C.4 Environmental factors that predispose to the development of frostbite include windchill,5 water immersion,6 high altitude,7,8 and chemical agent exposure.9 Physiologic factors that predispose to the development of frostbite include vascular dehydration,10,11 previous cold injury,12 alcohol or drug consumption,13,14 altered mental status/psychological illness, and inactivity.15
History
Historically, interest in frostbite has coincided with major military campaigns in which cold exposure has played a significant role. These include the American Revolution,16 the retreat of Napoleon’s Grand Army from Moscow,17 the First World War,6 the Second World War, and the Korean War. Ten percent of George Washington’s army perished during the winter campaigns of 1778 due to exposure to extreme cold.16 American soldiers suffered over 55 000 cases of cold injury in the Second World War,18 and over 8000 cases during the Korean War. The problem in civilian medical practice is small by comparison, but frostbite represents a recurring injury that deserves adequate knowledge of proper management.
Basic science/disease process
Pathophysiology
The blood flow to the skin is one of the most important means of heat regulation for the human body.19 The extremities account for approximately 50% of the total body surface area (TBSA) but are responsible for most of the protective vasoconstriction response to cold, because the skin covering the head and trunk has little capacity for vasoconstriction.20 Peripheral vasoconstriction is a physiologic response to cold exposure in order to preserve and maintain core body temperature.21 However, this physiologic peripheral vasoconstriction is interspersed with intermittent vasodilation, known as the hunting response. Such a response involves transient vasodilation of the vasculature of the extremities to increase blood flow through arteriovenous shunting in an effort to rewarm and perfuse the tissue. The hunting response occurs every 7–10 min until the body’s core temperature falls below 28°C, at which point the hunting response is lost.21 When the core temperature falls below 10°C, sensory nerve dysfunction occurs, leading to the loss of protective sensation. The loss of the hunting response and protective sensation in combination with subfreezing ambient temperature will lead to progressive tissue cooling and subsequent tissue freezing with ice crystal formation at –2°C.
Freezing phase
Tissue injury and death occur not only during the cooling/freezing process, but also during the rewarming/reperfusion process.22 During the cooling phase, prolonged vasoconstriction leads to cell ischemia, which activates the inflammatory cascade and initiates the release of a group of cytokines, including prostaglandins,23 bradykinins,24 thromboxane,23 and histamine.25 Inflammatory cells such as leukocytes26 and platelets27 are also recruited. Cell death during the freezing phase is believed to result from cellular dehydration.28 Slow cooling causes the extracellular fluid to freeze first. As the water in the extracellular space freezes and forms ice crystals, the water concentration in the extracellular space decreases and the extracellular space becomes more hypertonic than the intracellular space. As a result, water is drawn out of the cell by the osmotic gradient and leads to cellular dehydration. Cellular dehydration alters the protein and lipid composition of the cell membranes, making them less stable. The cellular pH also falls, which causes disruption of enzymatic activities. If the freezing process continues, ice crystals will form within the cell and cell membrane, causing disruption of membrane integrity and cell death. Rapid freezing (defined as a drop in tissue temperature >10°C per min) is less deleterious than slow freezing, because rapid freezing allows near simultaneous ice crystal formation in both the extracellular and intracellular space, therefore creating minimal osmotic gradient and minimal fluid shift.29 Furthermore, different cell types have different resistance to cold injuries. Nerve, cartilage, bone, and especially endothelial cells are more susceptible to freezing injury than are skin, fat, or connective tissue.3,4,26 This is why neuropathic pain is a common sequelae of frostbite injury, even without soft tissue loss, and also why frostbitten children can develop shortened or angular deformity in affected digits due to injury to the cartilaginous growth plates.
Rewarming phase
Although freezing and ice crystal formation may fatally injure a certain number of cells; the clinical significance of this direct injury to the soft tissue is thought to be limited. This was demonstrated when frostbitten skin transplanted to a new, vascularized recipient site survived, but developed necrosis if left in situ and not transplanted.30 The indirect cell injury that occurs during the rewarming/reperfusion process is believed to be more critical. The endothelial cells are very vulnerable during the rewarming stage and become highly permeable if injured.26 The increased permeability leads to significant interstitial edema as the blood flow is restored. In severe endothelial cell injuries, erythrocyte extravasation and perivascular hemorrhage occur and produce hemorrhagic blisters.26,29,31 The injured endothelial cells also detach from the basement membrane, leaving a raw vessel surface that is thrombogenic. The restoration of blood flow then leads to immediate platelet aggregation in the arterioles, followed by leukocyte aggregation and activation. These events result in thrombosis and progressive vascular obstruction. If thrombosis is limited to only some areas of the microcirculation, only partial soft tissue loss will occur. If the larger arterioles and arteries are affected, the entire limb is at risk of necrosis. The major determinant of tissue necrosis is the degree of endothelial cell injury and the extent of microvascular thrombosis during the rewarming process.
Diagnosis/patient presentation
Physical exam
Staging
The extent of the frostbite injury is determined by clinical examination and the final outcome of the injured sites. Frozen skin is cold, white, and firm to the touch. No descriptive or prognostic assessment can be made until the tissues have been rewarmed.32 The older classification defines injury grades as first- to fourth-degree, much like thermal burns.33 Currently, most clinicians classify frostbite as either superficial or deep.34 However, early clinical assessment of tissue viability has poor accuracy, and progressive changes in clinical appearance are anticipated.7 Definitive classification may not be possible for several weeks.
Radiographic studies
99mTc pertechnetate scintigraphy (99mTc bone scans) can be used to evaluate perfusion of bone and soft tissue and predict the final extent of tissue damage from frostbite. 99mTc bone scan images are acquired over three phases: the blood flow phase, the blood pool phase, and the delayed bone phase. Patterns of radioactive tracer uptake in each phase may show normal uptake, decreased uptake, or nonuptake. Several studies have shown that the delayed bone phase correlates best with the true extent of tissue injury and final outcome.35 The areas of nonuptake in the delayed bone phase will usually require amputation, while the areas of normal uptake typically heal uneventfully. The areas of decreased uptake are regions with marginal viability and may subsequently evolve into areas of normal uptake or areas of nonuptake.36 This explains why the predictive power of 99mTc bone scans is time-dependent. The diagnostic accuracy of bone scans performed at 48 h post-injury is only 84%, but can be improved when bone scans are delayed until 7–10 days post-injury, because during this time, the areas of marginal viability (decreased uptake) would have evolved and declared themselves as either viable (normal uptake) or nonviable (nonuptake).37 Interestingly, one study suggests that areas of nonuptake on bone scans 10 days after frostbite does not always lead to complete tissue necrosis.38 The possible explanation is that these areas may have marginal perfusion that is adequate to support tissue viability, but below the detection level of the 99mTc bone scans. Therefore, bone scans may be used to predict the extent of worst possible outcome, but if given enough time to heal and recover, the final amount of tissue loss may not be as extensive as predicted by the bone scans.
Magnetic resonance imaging and angiography (MRI/MRA) may be superior to 99mTc bone scans in the evaluation of frostbite due to the ability to visualize occluded vessels and line of demarcation between viable and nonviable tissue.39 However, the high cost associated with MRI/MRA, along with contraindications in patients having metallic implants, may limit their clinical application. Perfusion status of the frostbitten area can also be assessed with angiography. However, it is invasive and perhaps is better reserved only when thrombolytic therapy is also being considered.
Treatment/surgical technique
Nonoperative management
Field management
Initial treatment in the field should focus on protecting the affected extremity from mechanical trauma and avoiding rewarming. Cyclic thawing and cooling or inadequate rewarming can worsen a frostbite injury.22 The patient should be transferred to centers that are familiar and equipped to perform rapid rewarming.
Rapid rewarming
The core temperature of the patient should be measured, and if hypothermia is present, it should be treated appropriately with core rewarming. The core temperature should rise above 35°C prior to attempting rapid rewarming of the frostbitten area. Any attempt of extremity rewarming prior to this may cause a paradoxical core body temperature drop, as the peripheral vessel beds reopen and the cold peripheral blood returns to the core body. The ideal temperature for rapid rewarming has been experimentally determined to be between 40° and 42°C.40 Rewarming at this temperature results in the least amount of tissue injury. It is best achieved in a hydrotherapy tub. Rewarming should be continued for 15–30 min or until thawing is complete, which is marked by the red or purple appearance of the affected area, indicating the restoration of blood flow. This often coincides with extreme pain in 75% of the patients, and may require parenteral narcotic administration.29 Active motion during rewarming is helpful, but massage of the affected part is not recommended, as it may traumatize the friable frostbitten skin.
Adjunctive therapy
White or clear blisters contain fluid that is high in prostaglandin F2α and thromboxane B2.23 These cytokines promote vasoconstriction, leukocyte adherence, and platelet aggregation. They have been shown to mediate dermal ischemia in burns and pedicled flaps.23,41 The fluid should be aspirated but the deflated blisters should be left in place to prevent desiccation of the underlying wound bed. Ibuprofen, a specific thromboxane inhibitor, can be used to counter the effects of these vasoconstrictive cytokines and has been associated with the best tissue salvage.29 It is currently the standard pharmacologic treatment for frostbite. Topical aloe vera may also be applied to the affected area, as it is an effective thromboxane inhibitor and has been shown to reduce the degree of tissue loss.42 Systemic antibiotics are generally not indicated unless clinical infection is present.32 However, tetanus prophylaxis should be given or updated. Tetanus killed thousands of Napoleon’s troops during the invasion of Russia, and clinical tetanus infection in frostbite patients has been reported as recently as 1990.29,43
Operative management
Amputation
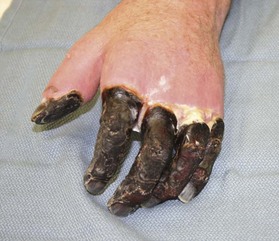
Distinguishing between viable and nonviable tissue in the early stages of frostbite injury is difficult, if not impossible. Therefore, every effort should be made to preserve as much tissue as possible while waiting for the frostbitten tissue to declare its viability, hence the classic adage of “freeze in January, amputate in July.” The only absolute indication for early debridement is uncontrollable infection.4 Mummification and black eschar occur at approximately 3–4 weeks post-injury.29 Amputation can generally be performed at that time, once there is a clear line of demarcation occurring between viable and nonviable tissue (Fig. 20.1).
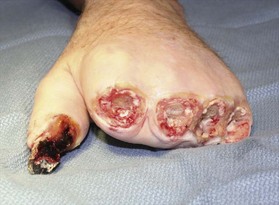
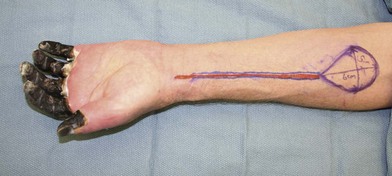
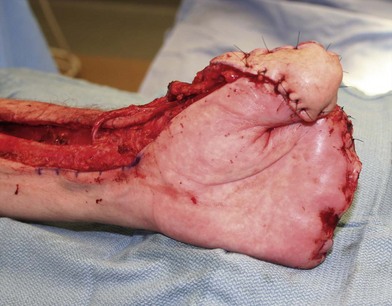
Amputation can be done without the use of tourniquet, which allows the surgeon to determine the level of viable tissue intraoperatively. Tissue is debrided until a bleeding wound edge is encountered (Fig. 20.2). The level of amputation should be conservative in order to preserve length and function.44 If the overlying soft tissue has sloughed but the underlying bony and tendinous structures are viable, a negative wound pressure dressing can be used to provide temporary coverage to prevent desiccation. Local or free tissue transfer can be utilized for length salvage (Figs 20.3, 20.4).45 However, function, and not length, ought to be the priority.
Length salvage
Advocates of early surgery utilize the results of 99mTc bone scan to guide surgical intervention. At 48 h post-injury, an initial bone scan is obtained. If there is any perfusion abnormality, the bone scan is repeated 72 h later. Areas with a persistent deficit in the delayed bone phase are treated with surgical excision of the nonperfused skin and soft tissues. The underlying tendon, nerve, and bone are covered with appropriate flaps to prevent desiccation. Follow-up bone scans demonstrated revascularization of structures covered by the flaps. This treatment algorithm limited the extent of required amputation with some length preservation in selected patients.46 However, there has not been any published long-term functional outcome study to demonstrate the efficacy of early surgical intervention when compared to initial observation followed by revision amputation at 4–6 weeks.
Outcomes/prognosis/complications
Common early complications of frostbite are infection and gangrene. Frequent late sequelae are residual pain and cold intolerance of the affected site.7 Less common complications include hyperhidrosis, pigmentation changes, and skin atrophy.47 Localized osteoporosis and pronounced subchondral bone loss have been reported as early as 4 weeks post-injury and as late as several months later.48 Physeal injury with subsequent growth disturbance has been observed in children.49–51 The most frequently affected site is the phalangeal epiphyses, following a pattern of decreasing frequency from distal to proximal. Direct injury to the vulnerable chondrocytes in the cartilaginous growth plate results in shortened digits or asymmetric growth of the digit leading to angular deformity. Radiographic evidence of premature epiphyseal closure may not be evident until 6–12 months after cold exposure.52 Long-term complications that involve joints and soft tissue injury may result in joint contracture and spontaneous joint fusion.
Secondary procedures
Sympathectomy
Surgical sympathectomy has been used to treat frostbite. It has been shown to be effective in decreasing the late sequelae of frostbite, including improved circulation, decreased hyperhidrosis and pallor, and reduced vasospastic symptoms and pain upon cold exposure.53–55 It has also been shown to expedite resolution of edema and the healing of ulcers.56,57 However, neither surgical sympathectomy nor chemical sympathectomy has been shown to decrease the amount of tissue loss after frostbite injury.58–60
Hyperbaric oxygen
The role of hyperbaric oxygen therapy in the treatment of frostbite remains unclear. In theory, it is attractive due to its ability to increase dissolved oxygen in the plasma, resulting in increased oxygen delivery to the tissue. It has been shown to accelerate capillary formation.61 Several anecdotal case reports describe improvement of wound healing and salvage of frostbitten digits with hyperbaric oxygen.62,63 However, there has not been any controlled clinical trial to demonstrate the therapeutic efficacy of hyperbaric oxygen therapy.
Thrombolytics
Since the major determinant of frostbite injury outcome is the severity of microvascular thrombosis that occurs after rewarming, thrombolytics are conceptually attractive because they potentially correct the pathology leading to tissue necrosis.1,64 Thrombolytic therapy involving either intravenous or intra-arterial tissue plasminogen activator (tPA) administration was found to improve the tissue viability after frostbite and decrease the need for amputation.65,66 The reported digit salvage rate for severe frostbite with perfusion deficits is approximately 75%.2 However, this involves invasive procedure with potential hemorrhagic complications. Its use has not yet been widely accepted.
Chemical injury
Chemical injuries are similar to thermal injuries, in that they are usually associated with damage to the skin only. However, the major difference is that some chemical may remain active at the contact site and cause continual tissue damage until neutralized. The level of injury depends on the duration of contact, and the nature and concentration of the chemical agent. Chemical burns represent only a small percentage of burn unit admissions. Yet more than 60% of these are work-related and have major occupational morbidity.67,68 Other exposures occur accidentally, in assaults, or during military conflicts.69 Alkalis are the most common chemical involved in cutaneous burns, but the most frequent single chemical agent involved is sulfuric acid.70 The silicon chip manufacturing industry is often associated with hydrofluoric acid burns.71,72
Many chemical burns involve the upper extremity and specifically the digits. Although the traditional measure of burn severity (percentage of total body surface area burned or %TBSA) is low compared with the usual burn population, chemical burns have a significant morbidity because the percentage of full-thickness burn is high and the patient’s hospital stay tends to be long.69 It is important to remember that while only about 3% of all burns are due to chemical exposure, 30% of burn deaths are due to chemical injuries.73
Basic science/disease process
Classification
The chemicals can be classified into six categories based on their mechanism of action: oxidizing agents, reducing agents, corrosive agents, desiccants, vesicants, and protoplasmic poisons.74 The pathophysiology of each category is briefly discussed below, and examples of representative agents commonly encountered from each category are described in further detail.
Oxidizing agents
Chromic acid
The active chemical in chromic acid is chromic trioxide. It is a pungent viscid yellow liquid. This hexavalent chromium, Cr(VI), is rapidly absorbed through the skin and reaches peak serum level by 5 h post-injury.75 Once in the circulation, Cr(VI) binds to hemoglobin, followed by parenchymal uptake by kidney, liver, bone, lung, and spleen within the first 24 h.76 Its toxic effect on the kidney may lead to renal failure.
Corrosive agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree