Fig. 2.1
Donor arm on the back table prior to dissection. Note the bruising after the removal of an intravenous line used in the donor prior to donation. The use of lines in the donor arm may compromise the vessels
Technique
Some surgeons have referred to hand transplantation as nothing more than a replant under ideal conditions. This might be true if you compare the allogeneic reconstruction of a guillotine amputation with ample pristine tissue from the donor to the replantation of a crushed and avulsed extremity with significant contamination and warm ischemia time. This highlights the major differences between the two techniques. In transplantation, there is plenty of donor tissue, and the surgeon is under less pressure to make a decision whether the damaged recipient tissue should be conserved. Another primary difference is that in transplants bone and tendon length must be carefully adjusted to match the recipient for a proper biomechanical function of the graft. Often in replantation, the bones and tendons are already at or near the appropriate length.
The specifics of the techniques of hand transplantation revolve around five different areas: (1) preoperative planning, (2) harvest and preparation of the donor graft, (3) preparation of the recipient and transplant of the donor graft, (4) immunosuppression and management of graft rejection, and finally (5) rehabilitation and therapy of the allograft to ensure the best possible function. The last two areas are beyond the scope of this chapter, and are mentioned only briefly. This chapter concentrates on the preoperative planning, harvest, and preparation of the donor, as well as the preparation of the recipient and attachment of the donor graft for transplantation of the upper extremity.
Preoperative Planning for the Operation
Once a patient has been accepted for hand transplantation , preoperative planning with respect to the approach and the donor graft will be required. If the transplant will require an unusual dissection of the recipient or donor, practice sessions in the cadaver laboratory should be scheduled and carried out. The primary differences between the surgery of replants versus transplants is that the level of amputation is different in the recipient and donor and the uncertainty of the length and quality of recipient vessels, nerves, muscles, and tendons remain until the surgery actually starts. Some centers perform imaging studies to obtain a roadmap of the recipient vascular anatomy by ultrasound [14]. Other imaging methods such as MRI or angiograms can also be used to prepare this roadmap. However, regardless of the imaging studies of the recipient prior to transplant, our experience has been that extent and quality of the patient’s vasculature and other soft tissues may look different from expected upon dissection. The surgeon will not know for certain what there is to work with until the recipient has been explored intraoperatively.
As part of the planning process, a transplant algorithm should be developed. This algorithm is used by the nurse coordinator and the lead surgeon to identify who should be called and when they should be called when a donor becomes available. At minimum, there should be two teams of surgeons, with at least two surgeons in each team who are experienced in performing replants. The first team’s primary responsibility is to go on the procurement run and harvest the donor graft and perform the dissection on the back table in the recipient’s operating room (OR). The second team’s primary responsibility is to perform the recipient dissection in preparation for the donor graft. If a bilateral transplant is being performed, four teams are needed. Hand transplantation is a long procedure and while a large team can reduce fatigue, care must be taken to avoid confusion and miscommunication about what has been completed when multiple surgeons are entering and leaving the OR. In our experience, teams of two surgeons rotated with each step, i.e., two for the dissection of the recipient, two for the osteosynthesis, two for the artery repair, etc. Each step takes approximately 2 h to complete (the osteosynthesis less time, and tendon and vein repair more time). Therefore, while in most cases multiple teams of two surgeons rotated throughout the transplant, once the donor dissection is completed, two teams could alternate between the steps of the procedure. This would give each team ample rest breaks and reduce surgeon fatigue. With this setup, a team of four surgeons would be sufficient to perform a hand transplant.
In addition, skilled anesthesiologists, nurses, and support staff are critical. Hypovolemia, metabolic acidosis, and reperfusion syndrome are risks of replantation [5, 7, 13, 32] and transplant patients must be carefully monitored by both surgical and anesthesiology teams. Recently, Caterson et al. have hypothesized that these ischemia reperfusion injury events may be a primary force causing graft injury and initiating rejection of the graft [8]. Preoperative planning, coordination, and communication with the surgical teams are of paramount importance to reduce ischemia time and operative complications .
Preparation of the Recipient
The recipient is prepped for surgery, has repeat laboratories, and has blood drawn for the donor crossmatch. The recipient is dosed with Benadryl and Solumedrol prior to infusion of thymoglobulin or alemtuzumab. Infusion of these induction agents may continue perioperatively. It is the experience at our center that the surgery is unaffected by these medications. Surgery is performed under general anesthesia and axillary block. Good collaboration with the anesthesiologist is necessary to maintain adequate circulatory status throughout the case. Quite often the patient comes to the OR relatively hypovolemic. The adjunctive use of alpha-mimetic agents for circulatory support should be avoided.
Harvest and Preparation of the Donor Graft
Acceptance of the donor occurs in stages; first, the parameters are communicated to the organ procurement organization (OPO) with respect to age, sex, skin tone, and general size. This information is used by the OPO coordinators to determine which donor families should be approached about hand donation. Additionally, the OPO reviews the donor with respect to infectious disease markers and serologic compatibility. Our program matches for blood type and the recipient must not have preformed antibodies against the donor. Both the donor and the recipient are HLA typed to determine Digital Signature Algorithm (DSA), but HLA type is not currently a consideration in accepting a donor. Finally, a radiograph of the entire donor upper extremity is sent to the hand surgery team for evaluation of whether the bone size is a good match with respect to length of the donor and the recipient. The hand surgeons also rule out any congenital or acquired bone or joint problems in the donor before accepting the graft.
The details of the recovery of the donor hand have been previously reported [2]. In most cases, recovery of the donor hand was performed prior to the recovery of the solid organs. A sterile tourniquet was placed on the donor upper extremity above the elbow, and a fishmouth incision is made at or slightly proximal to the elbow, and the elbow was disarticulated.
The order of dissection and tagging is as follows:
Cephalic and basilic veins (obtaining sufficient extra length for vein grafts, vessels should be procured as proximally as possible, the level of the tourniquet being the limiting factor).
Medial antebrachial cutaneous nerve (MACN) and the lateral anebrachial cutaneous (LACN) are dissected and tagged.
The brachial artery and veins are dissected and tagged.
Ulnar, median, and radial nerves are dissected and tagged.
The medial and lateral epicondyle muscles are elevated in a subperiosteal plane. The biceps, brachialis, and triceps tendons are transected.
An elbow capuslotomy is performed and the joint disarticulated. In the case of an above-the-elbow transplant, the arm would be disarticulated at the shoulder.
The donor graft is then prepared for transport to the recipient. If the donor and the recipient are in the same hospital, the donor graft would not be harvested until immediately before the recipient is taken to the OR to reduce ischemia time as much as possible. More frequently, the graft has to be prepared for transport at a separate hospital, which is often located in a different state. Currently, the graft is perfused with cold (4 °C) preservative solution. Our center prefers University of Wisconsin (UW) solution [3], but histidine–tryptophan–ketoglutarate (HTK) solution (Custodiol) was used in one case. The preservation solution serves a dual purpose of inducing hypothermia, which slows metabolism of the cells in the graft, and maintaining the intracellular electrolyte balance, thus extending the time before irreversible cell death occurs. The preservative is a proprietary mix that comes in bags and is connected to the graft via a cannulated artery (brachial artery in the cases harvested to date at our center) and then cold solution is flushed through the graft until the back flow is clear and the blood has been flushed from the graft. A drip of the UW solution is then attached and allowed to back drip through the veins. The graft is then dressed in gauze moistened with sterile saline and placed in a sterile plastic bag. The entire graft is placed in a cooler with ice and transported to the recipient site.
Our protocol allows for up to 12 h of cold ischemic time, starting from disarticulation of the extremity if our team is the first to procure, or from cross-clamp of the donor if the hand recovery team is following one of the solid organ recovery teams. Our group has harvested extremity grafts from a hospital a few blocks from the recipient OR, to as far away as Texas (about 950 miles from recipient hospital). The longest cold ischemic time from disarticulation to preparation of the donor hand in the OR has been 11 h. Of note, experimental evidence suggests a safe cold ischemia time might be much longer than 12 h. In a model of canine forelimb replantation , it was demonstrated that grafts could be held for up to 3 days and remain viable after replantation [4]. In this study, one forelimb of eight puppies and seven dogs was amputated, perfused with iced Collins solution, maintained at 4 °C for 72 h (78.5 h total anoxia), and replanted. Five animals were followed for 1 year to assess bone growth. Additional animals underwent bone labeling on days 1, 8, and 15, and were sacrificed at 22 days to assess osteocyte survival. Osteocytes survived replantation in all dogs and one puppy; most osteocytes died in two puppies. In five long-term puppies, central epiphyseal growth was disturbed, but the peripheral portions maintained nearly normal growth, with almost normal bone length being achieved at 1 year [4]. This data with carefully preserved limb replants with long cold ischemia time is in contrast to warm ischemia times which are extremely detrimental and contribute to ischemia reperfusion injury (IRI) and metabolic acidosis, especially when large muscles are reperfused [33]. Sabapathy et al. suggest that mid-forearm to wrist level replantation not be performed at all if warm ischemia exceeds 8 h, and that replantation is contraindicated with more than 6 h of warm ischemia for more proximal level replantation [28]. While keeping the allograft for 3 days before transplantation is not advocated, it is likely that cold ischemia time of longer than 12 h is possible in clinical VCA procedures. Reperfusion injury is a risk of limb transplantation. Proximal limb amputations with larger muscle mass are at higher risk of reperfusion syndrome, which can include multiorgan system failure and death [5, 7, 13, 32]. These risks are increased by more warm ischemic time. In a study of 14 patients who underwent replantation of an upper or lower limb, with 6 h or more of warm ischemia time, multiple reperfusion syndrome events were noted [33]. Four of the 14 patients suffered intraoperative hypotension with or without acidosis, and one child suffered acute bronchospasm following revascularization of the limb. One female patient died as a result of the hypotension and acute metabolic acidosis [33]. These risks will be mitigated in hand transplantation as there is some control of the donor warm ischemia time. It is the warm ischemia time that is most damaging to the neuronal and muscle tissues of the graft [5]. In the case of bilateral transplantation, the potential large amount of tissue and muscle mass may increase this risk. This is one rationale for in support of unilateral transplantation in clinical trials.
Surgical Dissection of the Recipient
Once the graft has been provisionally accepted by the transplant team, the recipient is notified, made nil per os (NPO), and arrangements are made to get the recipient to the hospital. At the time of graft procurement, the recovery team needs to ensure that lymph nodes are obtained from the donor so that a donor crossmatch can be performed. This test takes about 4 h to perform once the tissue typing laboratory has both the donor tissue and a sample of the recipient’s serum. If the clinical protocol lists the presence of donor-specific antibodies as an exclusion criterion, the surgery cannot proceed. With new procedures that allow the tissue typing laboratory to perform “virtual crossmatches,” some centers do not require a donor crossmatch to be performed prior to starting the dissection of the recipient. Our center has adopted the policy of requiring a prospective donor crossmatch if the recipient has known preformed specificities and a panel-reactive antibody (PRA). If the recipient is at low risk, i.e., has a PRA of 0 % and no known specificities identified on the virtual crossmatch, a retrospective donor crossmatch is requested to be performed on the next business day, and the recipient dissection can begin as soon as the donor graft is at the recipient hospital.
Upon arrival to the hospital, the recipient is rescreened for infectious disease markers, re-consented by the lead hand surgeon, and prepared for surgery. When it is clear that the graft will be acceptable for transplantation, the patient is given the required peri-transplant medications and immunosuppression , placed supine on the operating table and anesthetized.
Preparation of the recipient limb or limbs should occur at the same time as preparation of the donor graft on the back table. In cases of crush or burn injury, there may be significant scarring. Some centers use ultrasound imaging studies to map the veins prior to transplant [14], but this has not been found to be necessary in our experience. A tourniquet is placed on the recipient’s extremity that will receive the transplant. The incision used has been the mosaic or zigzag incision, to allow for tissue swelling, or in case that more skin is needed to close at the end of the case.
Careful dissection and identification of structures are paramount, and it is at this point the real status of recipient tissue is identified. In several of our cases, once the recipient tissues were fully exposed, it was found that the recipient arteries were smaller and shorter than expected; the tendons had retracted significantly, and were of insufficient length for straightforward reconstruction. Many times, fewer tendons were available than expected. In one case, despite a mid-forearm amputation, limited extensor tendons were available and the patient required a weave to give tenodesis. The surgeon should be aware that anatomic landmarks may be distorted due to the injury to the recipient, and the exact anatomy may be difficult to predict. Significant variation in anatomy and quality of the tissue should be expected. Versatility is required of the surgical team during this portion of the operation. As with the donor dissection, it is critical to label the identified structures. Our center keeps sterilized waterproof labels in the OR specifically for this purpose. Once blood flow is restored, the surgical field changes dramatically and blood and edema can make identification of small structures very difficult. Other centers use indelible ink on Esmarch bandages sewn to the structure with 2-0 silk [14].
Depending on the level of the amputation , the recipient’s extremity is dissected in such a manner that will preserve as much native tissue as possible for use in the transplant. This includes all vessels and nerves to the largest extent possible (Fig. 2.2). In general, the nerves are dissected and labeled first, then tendons, and then arteries and veins that will be used. The stump is then turned over and any available tissue such as the sensory branch of the ulnar and radial nerves is dissected and labeled (Fig. 2.3). Once all of the structures have been dissected and labeled, the radius and ulna in the case of a mid-forearm amputation are prepared for fixation with the donor graft. Communication between the teams is paramount, especially as length, and even availability of structures within the recipient may not be known until the actual surgery. Care must be taken that nerves, tendons, and vessels are of adequate length in both directions prior to bone fixation of the recipient and donor.
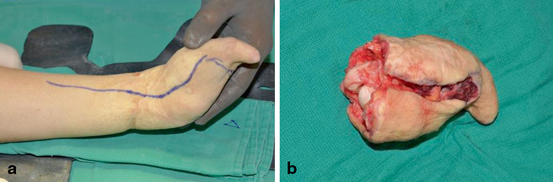
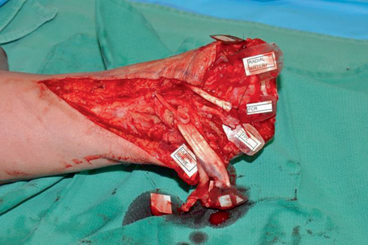

Fig. 2.2
Preparation of the dissection of the recipient, taking care to preserve as much of the recipient tissue as possible. Note in Fig. 2.2b that the digital nerve has been dissected as far as possible

Fig. 2.3
Labeling of the recipient structures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








