25 Cleft palate
Synopsis
 Normal speech is the primary goal of cleft palate repair; minimizing effects of maxillary growth is also important but ultimately secondary.
Normal speech is the primary goal of cleft palate repair; minimizing effects of maxillary growth is also important but ultimately secondary.
 Cleft palate repair prior to 1 year of age (ideally 9–10 months) results in better speech outcomes than later repairs.
Cleft palate repair prior to 1 year of age (ideally 9–10 months) results in better speech outcomes than later repairs.
 The levator veli palatini muscle is longitudinally oriented in the cleft palate patient. Realignment of the muscle to a transverse and posterior position in the soft palate is the key to a successful functional result.
The levator veli palatini muscle is longitudinally oriented in the cleft palate patient. Realignment of the muscle to a transverse and posterior position in the soft palate is the key to a successful functional result.
 Eustachian tube function is abnormal in cleft patients due to abnormal position of the tensor veli palatini muscle; this must be addressed in every cleft palate patient, usually with ventilating tubes.
Eustachian tube function is abnormal in cleft patients due to abnormal position of the tensor veli palatini muscle; this must be addressed in every cleft palate patient, usually with ventilating tubes.
History
The first recorded operative treatment of a cleft patient has been attributed to the period of the Chin (Tsin) Dynasty (c390 ad). The repair was of a cleft lip only, and no mention of cleft palate repair was made. Palatal clefts were confused with the more common fistulas resulting from tertiary syphilis and were not addressed surgically because of this association. The greater technical challenges of cleft palate repair no doubt presented a barrier to surgical treatment as well. Although the first known cleft palate repair was performed in the early 19th century, the introduction of anesthesia permitted a quantum leap in treatment as it did for many diseases.1,2
I always pronounced /th/ like /s/. As I grew up, the adjacent parts tended to close the defect, and by speaking slowly I articulated better. Thanks to the nasal quality of the language, I used to speak French more clearly than English. Nature is kind and trying to correct her mistakes, did her best to improve my unpleasant voice by contracting as strongly as possible the muscles of the nasopharynx … I believe that the nasal sound was due to faulty vibration of the air in its passage from lungs to nares, because the fissure prevented the soft palate from functioning.3
The same problem often occurs today when cleft palate repair is delayed until later in life.
After the acceptance of his doctoral thesis, Stephenson returned to his home in Canada and eventually became a principal founder of McGill Medical College, where he served as Professor of Anatomy, Physiology, and Surgery.4 Roux later published his account of the procedure in 1825, describing the simple suture closure of the surgically freshened edges, which he termed “staphyloraphie”.5
The notoriety surrounding the publication of Stephenson’s thesis angered Carl Ferdinand von Graefe (1787–1840), who proclaimed that he, rather than Roux, was the first surgeon to perform velar closure. Indeed, he had devised the surgery and performed it (unsuccessfully) before 1819. He had briefly mentioned the simple suture of the velum to the medical society of Berlin in 1816 but had failed to report it formally in the medical journals of the time. For von Graefe, “who appeared to have little interest in this procedure at the time, apparently deeming it of no importance, it now became the chief concern of his life.”6 Competition and national honor between surgical groups in Berlin and Paris split the European medical community on this topic. Von Graefe eventually reported a successful repair in 1820.7 The first successful closure of the soft palate in America was by John Collins Warren in 1820 in Boston.2
Johann Friedrich Dieffenbach (1792–1847) studied under von Graefe at the University of Berlin.8 Dieffenbach expanded the technique of soft palate repair to include closure of the hard palate. Dieffenbach’s palatoplasty method involved bringing together the cleft bony segments by a series of twisting silver or lead sutures passed through punch holes in the bony palate.9 He eventually designed and advocated lateral mucosal relaxing incisions to aid successful soft tissue closure. Later, following von Graefe at Charité Hospital in Berlin in 1840, he not only improved the technique of palatoplasty but also advanced techniques of local flap closure and transplantation. Dieffenbach’s possibly most important contribution to plastic surgery was the introduction of ether anesthesia to plastic surgical procedures.9 Dieffenbach had been introduced to anesthetic technique during a demonstration at Massachusetts General Hospital in 1846 and began applying it to routine surgical procedures before his death in 1847. He was so revered in Germany that he received an official state burial.
After the death of Dieffenbach, Bernhard von Langenbeck (1810–1887) succeeded to the position at the University of Berlin. Incorporating his extensive work with bone and periosteum of the extremities during the Franco-Prussian war, he was the first to describe the mucoperiosteal plane of dissection and to use its advantage in mobility to cleft palate closure.10 His technique, with various modifications, is still widely used today. The combination of Dieffenbach’s introduction of general anesthesia and von Langenbeck’s use of mucoperiosteal flaps ushered in the modern era of cleft palate surgery.
Basic science
Embryology
The embryology of maxillary and palatal development is reviewed in detail in Chapter 21. In broad terms, the failure of fusion of the frontonasal and maxillary processes gives rise to the cleft of the primary palate, which includes the lip, alveolar process, and the hard palate anterior to the incisive foramen. This results in a cleft in the typical location between the premaxilla and the lateral maxilla, on either one or both sides. The lateral palatal shelves fuse later than the primary palate, around 7–8 weeks’ gestation, as they rotate from vertical to horizontal orientation. This fusion proceeds from anterior to posterior, which helps to understand the spectrum of clefts of the secondary palate.
Anatomy
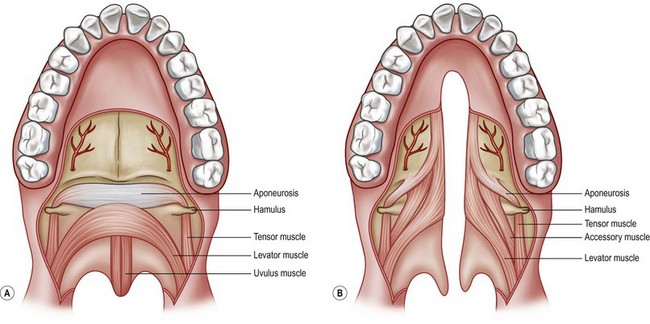
Careful anatomic evaluation of each patient is of paramount importance in considering palatoplasty. Anatomic variability within the broad diagnosis of cleft palate will influence the timing and sequence of surgical repair as well as the type of repair. Optimal functional results depend directly on accurate analysis of the available structures and understanding of their long-term significance to function and facial growth (Fig. 25.1).
In addition to being discontinuous across the cleft, the levator muscle runs more or less longitudinally along the cleft margin before it inserts aberrantly into the posterior border of the hard palate.11–13 This results in ineffective contraction and inability to close the palate against the posterior pharyngeal wall. Air escape through the nose during speech produces a characteristic hypernasal quality. In addition, aberrant levator positioning as well as an abnormal fusion with the tendon of the tensor veli palatini muscle is thought to impair the function of the tensor muscle in assists with Eustachian tube function and is thought to be contributory to cleft otopathology.14 A complete review of cleft otopathology is presented later in this chapter.
Ear pathology
Alt was the first physician to note a correlation between ear disease and cleft palate in 1878.15 Numerous studies have linked the presence of a cleft palate to abnormalities in Eustachian tube function. In multicenter studies, incidence of otitis media effusion has been found to be 96–100% in cleft patients, measured by both middle ear effusions on otoscopy and impedance testing and middle ear aspiration.16,17 In the cleft palate, impairment of tubal dilation is thought to occur from complex misalignment of the paratubal musculature.18,19 Both radiologic and manometric testing techniques have demonstrated abnormalities in active dilation of the eustachian tube.20 In addition, intrinsic abnormalities of tubal cartilage framework rendering a Eustachian tube more collapsible have been noted. Although anatomic studies show that the levator veli palatini muscle does not directly actively open the Eustachian tube orifice, it is likely to have a secondary effect by influence on the tensor veli palatini and also with passive position of the orifice. The levator and tensor do share a common tendinous insertion near the hamulus and pulley position around the hamulus. In the cleft patient, the levator is connected solidly against the rigid posterior hard palate; the pulley effect around the hamulus cannot be activated and impairs opening of the tube. In addition, some theorize that constant bathing of the tube orifice with oropharyngeal refluxed material leads to inflammation and obstruction of drainage. Other studies have demonstrated adenoidal tissue at the level of the tubal dilator that could potentially contribute to mechanical obstruction.21 Anatomic paratubal abnormalities and risk for serous otitis media and chronic audio-logic sequelae are present, regardless of cleft type, although Pierre Robin sequence has been postulated to be at even higher risk for hearing loss because of potential for concomitant ossicular malformation.22
Chronic obstruction of drainage leads to serous otitis media, and long-standing effusion can result in hearing loss. Estimates are 20–30% incidence of pure tone hearing loss in cleft patients by audiography23; decreased hearing has been found in as many as half of cleft palate patients by other authors.24 Untreated children with clefts and severe effusions may have total deafness. Whereas hearing loss is significant in any child, it may be even more so in a cleft patient in whom speech development may be abnormal.
It has long been suggested that closure of the palate reduces risk of permanent hearing loss. In retrospective studies, children who had undergone palatoplasty had significantly lower incidence of permanent hearing loss than did children with unrepaired cleft palates.24 Although still controversial, palatoplasty is thought by most surgeons to reduce risk of chronic serous otitis media and hearing loss, although not by universal agreement. Nevertheless, serous otitis persists in most cleft patients for several years after palate repair, and myringotomy with placement of ventilating tubes remains the mainstay of treatment for this difficult problem.
Patient presentation
Cleft palate with cleft lip and alveolus
Although the primary palate and the secondary palate form at different stages of embryonic development, cleft palate is most commonly seen in combination with cleft lip. The alveolar portion of the cleft lies between the maxillary lateral incisor and canine tooth roots. This results in malposition of the maxillary lateral incisor and cuspid in both the deciduous and permanent dentition.25,26 The maxillary lateral incisor on the cleft side is absent in 80–90% of cleft patients; when present, it may be smaller than the contralateral tooth or significantly dysmorphic26–28 Absence of other teeth is not uncommon,29 but it may be related to the surgery itself because adults who have not been operated on do not show the same patterns of hypodontia.30 Asymmetry is common, resulting in alterations in first maxillary molar position.31–33 In comparison to noncleft individuals, patients with unilateral cleft lip and palate have overall reduction in crown size34 and delay of eruption of permanent dentition.35 These findings support the theory of global dental growth potential disturbance associated with clefting. Interestingly, eruption of deciduous dentition is not significantly delayed.36
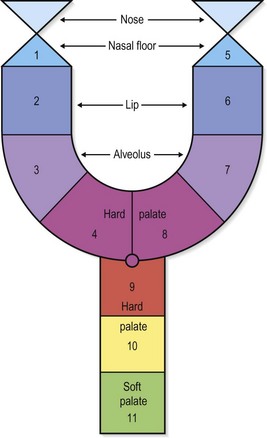
Kernahan proposed a standardized reporting form to distinguish the variable presentations of cleft lip and palate, shown in (Fig. 25.2).
Submucous cleft palate
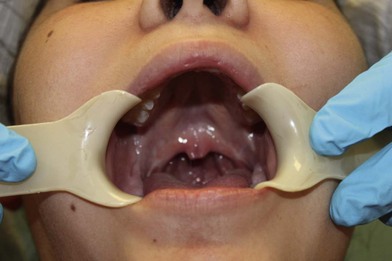
Submucous cleft palate occurs when the palate has mucosal continuity but the underlying levator palatini muscle is discontinuous across the midline and longitudinally oriented, similar to the muscle anatomy in overt clefts of the palate. Calnan’s classic triad of a midline clear zone (zona pellucida), a bifid uvula and a palpable notch in the posterior hard palate is diagnostic of this condition. With contraction of velar musculature, a distinct midline muscle diastasis may be seen (Fig. 25.3).
The significance of a submucous cleft may be difficult to assess clinically; the child with submucous cleft palate is often undiagnosed in infancy. In a study screening a large population of schoolchildren in Denver, the overall incidence of submucous cleft palate in the population was found to be 1 : 1200.37 Another similar cohort study reported that 45–55% of patients with isolated submucous cleft palate were symptomatic with regard to speech, serous otitis media, or hearing loss.38,39 In a large series of children with submucous cleft palate in Mexico, examination with fiberoptic nasendoscopy showed about one-third to have velopharyngeal insufficiency.40 Other attempts to identify risk of speech problems with submucous cleft palate have been problematic because referrals to cleft palate clinics are usually based on the identification of speech issues rather than of the submucous cleft itself. Therefore, an infant identified with submucous cleft palate need not routinely undergo repair because a significant number of individuals with submucous cleft palate will not develop velopharyngeal insufficiency. Rather, these patients should be closely monitored with serial speech evaluations and audiometric surveillance.
Patients who present with velopharyngeal insufficiency and submucous cleft palate on examination require full evaluation, including speech evaluation and endoscopy.41 Even in the absence of obvious findings on clinical examination, anatomic abnormalities are found in most patients (>90%) at the time of surgery42,43; thus, the so-called occult submucous cleft palate is simply one that is less obvious on clinical evaluation. There will still be rare patients, however, who have true palatopharyngeal disproportion and present with velopharyngeal insufficiency without any cleft, submucous or otherwise.
Corrective surgical technique for submucous cleft palate is focused on anatomic correction of the velar muscle diastasis. Although pharyngeal flaps and sphincter pharyngoplasty have been proposed as primary means of treatment,40 most surgeons focus on repair of the abnormal levator muscle position.44 The Furlow double opposing Z-plasty (see below) is an ideal procedure for these patients because there is no width discrepancy to be overcome.45 This avoids the potential for nasal obstruction and sleep apnea in these patients if a pharyngeal flap is used.
Pierre Robin sequence
Pierre Robin described the triad of micrognathia, glossoptosis, and respiratory distress.46 Of the children diagnosed with Pierre Robin sequence, 60–90% have cleft palate47,48; the palatal cleft is usually isolated to the velum and can be V shaped or, more typically, U-shaped.49,50 In the past, this has been thought to be secondary to hyperflexion of the head in utero with resultant displacement of the tongue between the palatal shelves, preventing their fusion. More recently, extensive analysis of multiple syndromes associated with Pierre Robin sequence has delineated genetic associations, indicating that the etiology may not be such a simple mechanical event.51–53 Infants with Pierre Robin sequence also have increased incidence of associated anomalies, particularly cardiac and renal problems.54
Newborns with Pierre Robin sequence may have severe respiratory and feeding difficulty because of the posterior displacement of the tongue. Initial treatment consists of placing the child prone and use of gastric lavage feeding tubes to push the tongue forward.55 Nasal airways have been used for the same purpose with reported success rates of 80–90%.56 If these conservative measures fail, surgical management of the airway may be required. A tongue-lip adhesion has been used as an alternative to tracheostomy and is generally effective.57,58 More recently, mandibular distraction osteogenesis has been used in neonates with success in averting tracheostomy, although long-term outcomes in regard to mandibular growth and dental development are not yet available.59 In all cases, if management is focused on the upper airway, bronchoscopy should be performed to rule out any intrinsic subglottic problems (e.g., laryngomalacia) that might necessitate tracheotomy.
Palatoplasty in children with Pierre Robin sequence must be carefully timed with growth of the child, particularly the mandible. There is some controversy about “catch-up” growth of the mandible in children with Pierre Robin sequence; basically, there is good documentation of extra growth in the first year of life,60,61 but growth is subsequently commensurate with that of normal children. Children with syndromes tend to have less growth than nonsyndromic cases. Late cephalometric evaluation has consistently shown that the children with Pierre Robin sequence have smaller mandibles than normal.62 Closure of the palate narrows the effective area for respiration and can lead to respiratory distress. If the mandible attains reasonable size in the first year of life, palate repair can still be performed safely before 1 year of age. In the rare patient who has previously undergone tracheostomy, the palate should be repaired before decannulation. The risk of airway compromise after palatoplasty reaches 25%, with an emergent tracheostomy or reintubation rate of 11% at one institution.63
Syndromes
Multiple malformations or syndromes have been found frequently in cleft patients.64 Cleft palate without associated cleft lip has been reported to be associated with a syndrome in as many as 50% of cases, while cleft lip and palate together have an incidence of syndromes of about 30%. Van der Woude syndrome is associated with a mutation in the interferon regulatory factor 6 (IRF6) gene that also causes popliteal pterygium syndrome; this is an autosomal dominant syndrome associated with lower lip sinus tracts (“lip pits”), and has variable penetrance including the full range of cleft lips as well as palates.
22q chromosomal deletion
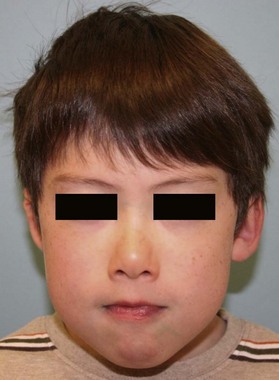
Velocardiofacial syndrome, which is associated with a 22q chromosomal deletion, is detected by fluorescent immunohybridization (FISH). These children have a characteristic “bird-like” facial appearance, soft palate dysfunction, developmental delay, and various cardiac conditions (Fig. 25.4). The same deletion gives rise to DiGeorge syndrome with associated B-cell and immune dysfunction; these children all need appropriate immunology referral and follow-up. The majority of these children does not have overt cleft palate and may not even have submucous clefts; usually the velar dysfunction is related to absence of movement of an otherwise normal soft palate. This may complicate decision-making, regarding surgery for speech in these patients.
Growth
At birth, the average weight is the same for cleft and unaffected newborns.65,66 However, cleft infants have been shown to exhibit poor weight gain in early infancy. Studies observing infants with cleft lip and palate show initial growth retardation by the time they undergo surgical lip repair. When the same children reach the age for palatoplasty, they have significantly lagged on the growth curve. However, longitudinal studies show that after repair of the palate, average growth returns to normal compared with unaffected children by the age of 4 years. Stratification of risk within the longitudinal cohort shows pronounced growth retardation in associated syndromes and also in children with isolated clefts of the secondary palate.67,68
Children with orofacial clefting stabilize and continue normal growth to at least 6 years of age, with no statistically significant differences in height and weight when compared to unaffected children. In later childhood, however, average weight and height of children with cleft appear to diminish compared with those of control subjects. In a Danish study of skeletal maturity assessing both body height and radius length as an index of growth in male cleft patients, onset of puberty was found to be delayed on average by 6 months, and the velocity of skeletal growth during puberty was blunted. However, duration of puberty and pubertal skeletal growth were prolonged an average of 1 year, resulting in final attained body height and radius length the same as those of control subjects.69
Numerous causes are thought to contribute to these differences in growth patterns. There are certainly feeding difficulties early in life before palate repair,70 but it has also been suggested that intrinsic growth disturbances may be responsible for slow postnatal growth.71 The increased frequency of ear and airway infections has been implicated in early growth retardation,65 as have the multiple operative procedures that these children undergo.70 Adding more confusion to the etiology is the fact that growth hormone levels may be diminished in cleft children. In summary, any growth disturbance is likely to be of multifactorial origin throughout infancy, childhood, and puberty. Fortunately, families can be counseled that most children attain norms of height, weight, and development.