Chest Wall Reconstruction
Joseph N. Carey
Leo R. Otake
Anthony Echo
Gordon K. Lee
HISTORY
Chest wall reconstruction became more formalized as a result of reconstructing mastectomy defects. Tansini is credited with the first latissimus dorsi flap for reconstruction of a mastectomy defect in 1896. He developed fasciocutaneous, muscle, and musculocutaneous flaps for radical mastectomy defects. The surgical treatment of breast cancer at the time included resection of the breast, the pectoralis major, and the axillary contents. Halsted, who first performed the procedure in 1882, proposed skin graft closure or healing by secondary intention. Tansini’s experiments with random fasciocutaneous, muscle, and eventually pedicled musculocutaneous flaps gave him experience with partial and full flap necrosis. His tribulations led him to describe the concepts of blood flow in flaps and to conclude that the musculocutaneous latissimus flap was the most reliable method of reconstructing a mastectomy defect.
Tansini’s concept of mastectomy closure was lost for many years, as the Halsted method of breast cancer treatment was adopted, and admonitions about the use of flaps in cancer treatment were heeded. As the use of muscle and myocutaneous flaps was popularized in the 1970s by Mathes and Nahai and Bostwick, however, the superiority of flaps in the closure of mastectomy defects was demonstrated.
Similarly, as the surgical treatment of thoracic diseases evolved, large chest wall defects were created, presenting reconstructive challenges. Arnold and Pairolero in the 1970s and 1980s made substantial contributions using several muscle flaps (including the external oblique, pectoralis major, and latissimus dorsi) and omentum for chest wall reconstruction.
Modern-day chest wall reconstruction uses the gamut of the reconstructive armamentarium, including negative pressure wound therapy (Chapter 3), local flaps, pedicled flaps, and free tissue transfers. Alloplastic and prosthetic materials are also frequently used and their use has increased in recent years with the advent of biological prosthetic materials. This chapter focuses on the description of chest wall wounds, and the algorithm and materials available to the reconstructive surgeon to solve the problems associated with ablative surgery, trauma, and infection of the chest wall.
CHEST WALL ANATOMY AND FUNCTION
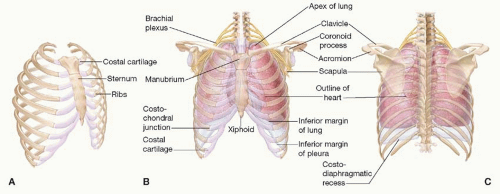
The chest wall consists of muscle, cartilage, and bone arranged in a conical fashion and consisting of an apex and a base (Figure 92.1). The junction of the first thoracic vertebrae, the first ribs, and the manubrium forms the apex or “thoracic outlet.” The base is formed by the diaphragm and its attachments to the inferior ribs, the xiphoid process, and the spine. The anterior surface of the chest wall consists of the sternum and its cartilaginous attachments to the anterior ribs. The chest wall is connected to the upper extremities anteriorly via the sternoclavicular joint and posteriorly through the soft tissue attachments of the scapulae.
The arterial supply to the chest wall consists primarily of paired intercostal arteries that originate from the aorta posteriorly, run through the intercostal spaces, and join the internal mammary arteries. The secondary arterial supply originates from the subclavian and axillary arteries via thoracoacromial, lateral thoracic, and thoracodorsal branches. The venous drainage parallels the arterial supply, however, in the posterior mediastinum the intercostal veins terminate in the azygous system. Paired intercostal nerves corresponding to the anterior rami of the T1 to T11 thoracic nerves travel with the neurovascular bundles in the intercostal spaces and provide motor innervation to the intercostal muscles as well as sensation to the overlying skin (Chapter 4).
The functions of the chest wall include (1) sturdy protection of the thoracic viscera; (2) assistance with respiratory function via muscular contraction and structural stability; (3) symmetric attachment of the upper extremity musculature and stabilization of the shoulder joint; and (4) symmetric attachment of the breasts.
Respiratory function depends on chest wall musculature and the stability of the rib cage. The chest wall muscles are arranged in three layers similar to the abdominal wall. Contraction narrows the intercostal spaces and changes the thoracic volume to change the intrathoracic pressure to effect air movement.
ETIOLOGY OF CHEST WALL DEFECTS
Chest wall deformities occur from a variety of causes, including trauma, tumor extirpation, infection, and iatrogenic injuries such as radiation. The origin of the defect, the age of the patient, and concomitant comorbidities all affect the reconstructive decisions.
Trauma. The most common cause of chest trauma in the United States is blunt trauma associated with motor vehicle accidents (MVAs). An estimated 7% of MVAs result in serious thoracic injury and 20% of all trauma deaths involve thoracic injury. In addition, penetrating, blast, or burn injuries may necessitate chest wall reconstruction.
Tumor. Tumor extirpation results in chest wall defects that range from small to massive. Among the most common neoplastic causes for chest wall resection are breast carcinoma and soft tissue sarcoma. However, extrathoracic extension of thoracic visceral tumors and primary bone and cartilage tumors are also causes of large chest wall defects. The specific
adjuvant treatments for each type of tumor are taken into consideration, including the potential need for chemotherapy and radiation.
adjuvant treatments for each type of tumor are taken into consideration, including the potential need for chemotherapy and radiation.
Infection. Infections involving the chest wall and thoracic cavities are common indications for reconstruction. Origins of intrathoracic infections include empyema, bronchopleural fistula, pneumonia, and surgical site infections following thoracic surgery. Mediastinal sepsis and sternal osteomyelitis may occur following heart surgery and require prompt and complete debridement and coverage.
Radiation. Radiation treatment of tumors in the chest wall, most commonly breast cancers, results in difficult to manage wounds that require resection and coverage with vascularized tissue. Osteoradionecrosis of ribs and the sternum may occur years following treatment of carcinomas and lymphomas, and results in recurrent infection and drainage.
Congenital. Congenital chest wall defects requiring reconstruction are most commonly pectus excavatum, pectus carinatum, and Poland’s syndrome (Chapter 64). Other conditions include lymphatic and vascular malformations.
Trauma of the Chest Wall
Rib fractures indicate significant chest trauma and may be associated with intrathoracic and intra-abdominal injury. Fractures of three adjacent ribs in two or more places may result in a flail chest and paradoxical motion, and may lead to respiratory compromise. Blast and electrical injuries may induce zones of injury not immediately apparent in the acute setting and require close cardiac and respiratory monitoring.
Treatment. After stabilization of life-threatening injuries, patients with flail chest may require operative stabilization of rib fractures. While the mainstay treatment of rib fractures is nonoperative, rib plating systems are currently available for the stabilization of rib fractures in a flail chest segment. Rigid fixation systems contain a standard plate and screws, as well as intramedullary rods.
In traumatic wounds, coverage after debridement of nonviable tissues is approached similarly to infections and tumor defects (see below).
Tumors of the Chest Wall
Primary malignancies of the chest wall may be classified into eight main categories: muscular, vascular, fibrous and fibrohistiocytic, peripheral nerve, osseous and cartilaginous, adipose, hematologic, and cutaneous. The diversity of malignancies and attendant surgical extirpation may result in significant defects. Reconstruction must also take into account postoperative oncologic therapy such as radiation.
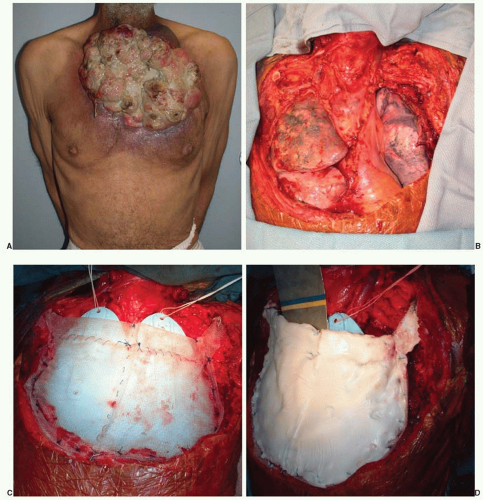
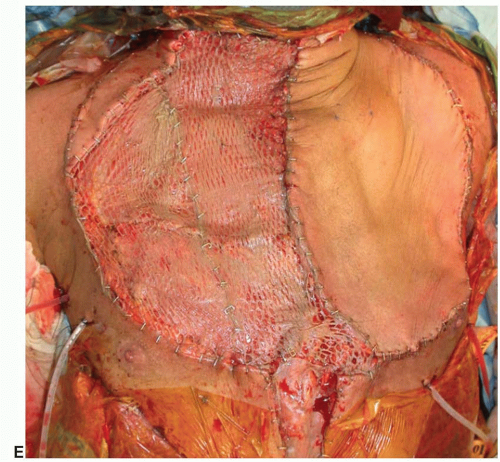
Treatment. After extirpation of the tumor, the dimensions and components of the chest wall requiring reconstruction are evaluated. Restoration of pleural cavity integrity as well as protection of intrathoracic structures may be required. Classic teaching recommends skeletal reconstruction for defects involving four or more ribs or greater than 5 cm in diameter; however, this varies depending on the location of the defect.
An option for skeletal reconstruction includes the so-called methylmethacrylate and synthetic mesh “sandwich.” The methylmethacrylate is molded into the desired shape of the defect, and Marlex or Prolene mesh is placed on each side of the construct and sutured together. The methylmethacrylate and mesh sandwich can be sutured to the surrounding structures. Some patients experience pain with respiration since the methylmethacrylate is much more rigid than the chest wall. This method of skeletal reconstruction provides protection to the underlying cardiac and pulmonary structures and can be used for even large defects in tandem with soft tissue flaps (Figure 92.2).
Posterior and superior chest wall defects may not affect ventilation as much as anterior defects. In these cases, skeletal reconstruction may not be necessary; therefore, a variety of other products, both synthetic and biologic, may be appropriate. Synthetic products such as Gore-Tex, Marlex, and Prolene mesh may be used for smaller lateral defects. Synthetic mesh can be problematic in the lower chest wall since the mesh may come into contact with the intra-abdominal contents and lead to bowel adhesions or even fistulae (Chapter 93). Biological products, such as human allograft dermis and xenograft dermis, offer other options for reconstruction where rigid stability is not mandatory. They are not likely to cause adhesions with the viscera and may tolerate bacterial contamination better than a synthetic product since they may become revascularized by the surrounding host tissue. Although the discussion of biologic materials is beyond the scope of this chapter, surgeons have continued to expand applications in complex defects and wounds. Both biologic and synthetic mesh do not provide the same level of rigidity as methylmethacrylate or titanium mesh, but they do provide additional stability to the chest by spanning the defect.
Infections of the Chest Wall and Thorax
Empyema and Bronchopleural Fistula. A bronchopleural fistula and pleural empyema (pus in the pleural cavity) are complications of pneumonectomy that carry significant morbidity and mortality. As a first step, drainage of the infectious empyema is performed, and depending on its severity, may necessitate a delay in the reconstruction. Often the thoracic surgeon manages the empyema and requests help from a plastic surgeon for the transfer of flaps to reinforce the bronchial stump closure or to obliterate the pleural cavity.
Treatment
Eloesser Flap. To adequately drain the empyema cavity, a variety of procedures have been described. The Eloesser flap, originally described in 1935 for the drainage of tuberculous empyemas, externalizes the empyema. A 2-inch random-patterned fasciocutanous flap is created on the chest wall at the level of the empyema, a segment of rib is resected, and the flap is sewn to the pleural lining (Figure 92.3A). This allows for continual drainage of the empyema.
Clagett Procedure. The Clagett procedure involves creation of a window thoracotomy and the packing of the wound with
antibiotic-soaked dressings that are changed every 48 hours. Once the pleural space contains healthy granulation tissue, the cavity is completely filled with antibiotic solution and the chest wall is closed in layers.
antibiotic-soaked dressings that are changed every 48 hours. Once the pleural space contains healthy granulation tissue, the cavity is completely filled with antibiotic solution and the chest wall is closed in layers.
Thoracoplasty. Obliteration of the pleural space is necessary if the remaining lung does not fill the hemithorax. A postpneumonectomy syndrome of tracheal deviation, inspiratory stridor, and exertional dyspnea may develop. Historically, a thoracoplasty was performed, where the skeletal support was removed, leading to the external collapse of the hemithorax (Figure 92.3B). While this procedure addressed the dead space from the pneumonectomy, it was quite morbid and disfiguring.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree