CHAPTER 15 Filaria
KEY POINTS
Adenolymphangitis is an important clinical manifestation of lymphatic filariasis; recurrence contributes to the progress of the disease.
Meticulous compliance with nonsurgical methods of treatment is essential to avoid surgery or prevent recurrence after surgery.
A combination of physiologic surgery, such as a nodovenous shunt or a lymphaticovenous shunt, with an immediate reduction or debulking procedure with skin grafting is a very effective treatment method.
Lymphatic filariasis has no cure.
Approximately 120 million people worldwide are at risk of contracting filariasis, and 70 million people have established filariasis, of whom 40 million have lymphedema. Medical and paramedical professionals and health planners need to understand this disease and attempt to prevent and decrease morbidity by various means, including surgery. The World Health Organization is working toward elimination of lymphatic filariasis by 2020. In the meantime, patients with clinical manifestations require lifelong treatment. In the future, earlier detection and treatment may reduce the severity of the disease but will not eliminate the problem.
Lymphatic filariasis is one of the most incapacitating of chronic diseases. Previously, no treatment was thought possible. Ancient sculptures and scriptures depicted lymphatic filariasis of the lower limb; these are currently seen in many temples in India. According to Manusmriti (300 BC) from Hindu mythology, the disease was considered a result of karma, a retribution for actions committed in a previous life. Some primitive treatments were performed. As science and technology progressed, the organism and its mode of transmission from mosquitoes to humans were identified. Initially many medical and surgical treatments were attempted, but invariably they were unsuccessful, and lymphatic filariasis became a neglected tropical disease.
Pathogenesis of Lymphatic Filariasis
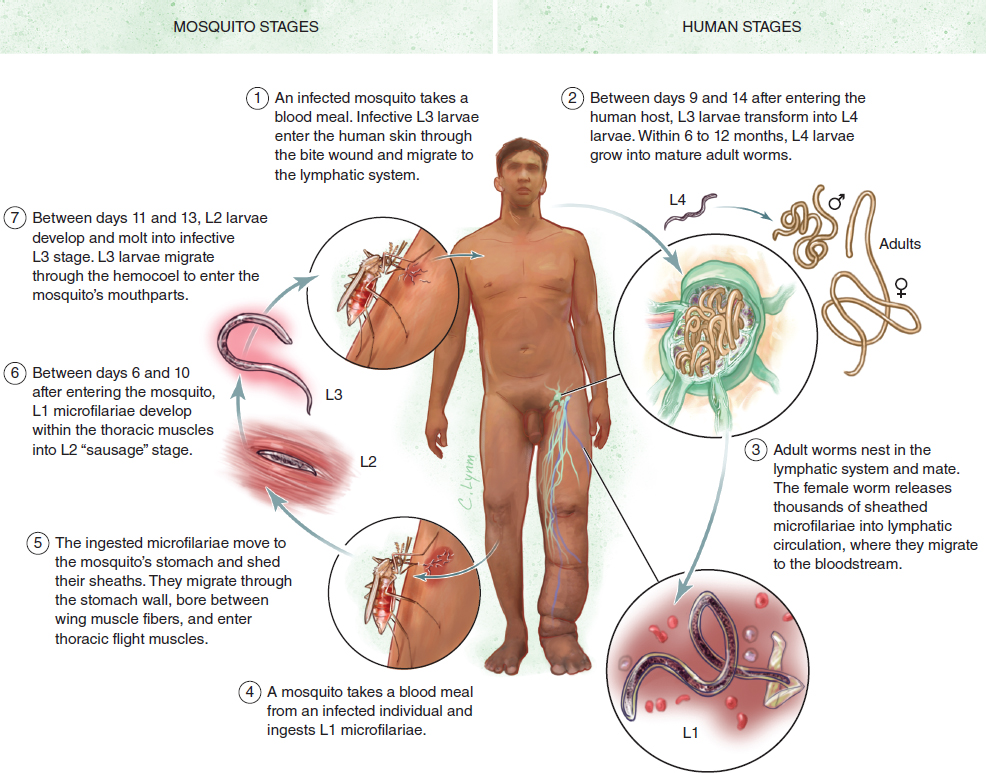
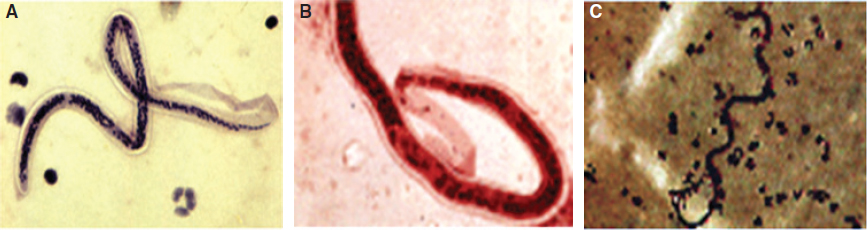
Lymphatic filariasis is a vector-borne disease of tropical countries caused by infection with filarial worms transmitted from the Culex species of mosquitoes (Fig. 15-1). The breeding of these mosquitoes is associated with aquatic plants such as Pistia stratiotes (water lettuce) and Salvinia auriculata (butterfly fern). The infective mosquitoes harbor the larvae (microfilariae), which enter the human host through bite wounds and migrate into the lymphatic system, where they develop into adult male and female worms. These worms—most commonly Wuchereria bancrofti (Fig. 15-2, A), Brugia malayi (Fig. 15-2, B), and Brugia timori (Fig. 15-2, C)—invade the lymphatics, leading to pathologic changes culminating in filarial disease manifestations. 1 In mainland India, the causative nematodes are mostly W. bancrofti (see Fig. 15-2) and B. malayi, which are transmitted by specific genera of mosquitoes that include Culex quinquefasciatus, Anopheles, and others. B. malayi infection is now reportedly restricted to rural areas of South India.
The worms live in the lymph vessels and lymph nodes by making nests in the dilated lymphatics. The term worm nests refers to dilated lymphatic vessels with the characteristic movement pattern of worms, as evidenced on ultrasound examination. This early pathologic state predisposes the system to lymph dysfunction by causing incompetence of the unidirectional valves.
Lymphatic filariasis is characterized by the presence of live adult parasites in the lymphatic system, with larvae (microfilariae) in the blood at certain times of the night (generally) in the early stage of the development of the lymphedema. The presence of microfilariae indicates an early stage in the life cycle in which the adult worms live in the lymph vessels and lymph nodes while they release microfilariae into the bloodstream of the host. The presence of microfilariae in the host bloodstream is called microfilaremia. This happens after the worms mate and the females produce millions of microfilariae, which migrate to the blood circulation. The sheathed microfilariae begin to appear in the blood circulation in 6 to 12 months after infection. They remain in the arterioles of the lungs during the day and emerge into the peripheral circulation at night. The periodicity of microfilariae coincides with the biting activity of the vector. They are taken up by blood-feeding vectors that spread the disease.
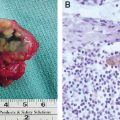
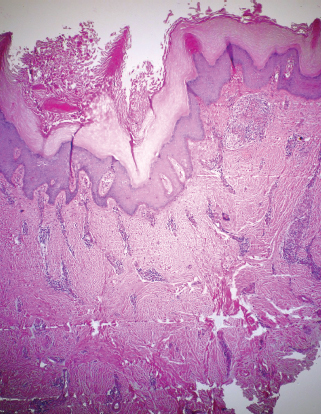
Histopathologically, the filarial tissue shows hyperproliferation of keratinocytes, focal acantholysis, an accumulation of lymphocytes at the epidermodermal junction, and profuse pericapillary and perivenular mononuclear infiltrations (Fig. 15-3).
Manifestations of Lymphatic Filariasis
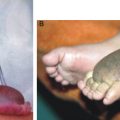
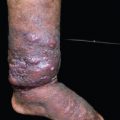
The manifestations can be categorized as acute and chronic disease states (Table 15-1). Acute manifestations are comprised of adenolymphangitis, epididymoorchitis, and funiculitis, whereas chronic manifestations include lymphedema, hydrocele, elephantiasis, chyluria, chylothorax, chylascites, lymph scrotum, and tropical pulmonary eosinophilia. Advanced stages of lymphedema are characterized by increasing dilation and tortuosity of the lymphatics, endothelial proliferation, formation of new lymph channels, and obstructive changes with dermatosclerosis and warty lesions. 2 Genital manifestations include filarial scrotum, ram’s horn penis, genital vesicles, and edema. Atypical lymphatic filariasis manifests as fleeting joint pains and lymphangitis (string sign). It can affect the breast, gluteal region, abdomen, and suprapubic region as isolated lesions. Manifestations are most commonly seen in the lower limb, more often in females.
Four grades of lymphedema have been described, depending on the severity of involvement and the quality of pitting. 3
Grade 1: Edema appears and disappears spontaneously and is pitting and uniform in size.
Grade 2: Edema is persistent, pitting, and uniform.
Grade 3: Edema is persistent, nonpitting, and uniform.
Grade 4: Giant lymphedema develops, with complications such as ulcers, warty growth, and loss of limb shape (elephantiasis).
Any breach of skin integrity of the affected region (for example, from injury, fungal or bacterial infection, or even eczema) favors entry of pathogenic bacteria into the tissues, leading to acute attacks of adenolymphangitis, which is commonly seen in filarial limbs. 4 , 5 Adenolymphangitis is one of the important clinical manifestations of lymphatic filariasis. Recurrence contributes to the progress of the disease and has important socioeconomic implications, because it affects a patient’s ability to work. 6
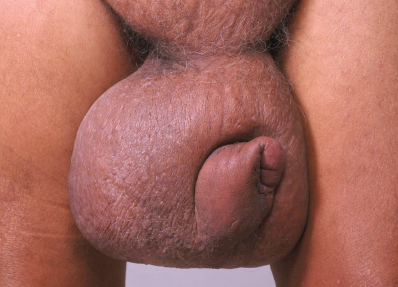
Lymphedema of the extremities is a common chronic manifestation of lymphatic filariasis that results in elephantiasis as it progresses. Elephantiasis refers to massive swelling of the lower limbs caused by repeat attacks of filarial lymphangitis over several years. The limb becomes grossly enlarged, resembling the foot of an elephant. Repeated inflammatory reactions cause vessel dilation and thickening. In the advanced stages of lymphedema, the skin is thickened and thrown into folds, often with hypertrichosis, black pigmentation, nodules, warty growth, and intertrigo in the webs of the toes, with nonhealing ulcers. This usually involves the lower limbs, commonly unilaterally. The male genitalia are also commonly affected (Fig. 15-4). Vulval elephantiasis has been reported. 7
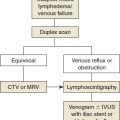
Diagnosis
Patient history is essential for diagnosing lymphatic filariasis, because it provides information about the causes. Careful clinical examination of the skin color, texture, and other changes is helpful for staging the disease. Circumferential measurements at fixed points of the upper and lower limbs are documented. The patient’s height and weight are recorded.
Several diagnostic tools are available, including the following:
An immunochromatographic test can be conducted quickly and easily at the bedside to test for lymphatic filariasis. The test is highly sensitive for W. bancrofti.
Ultrasonography can be used as a screening test in endemic areas. It can reveal moving adult worms in the scrotum or breast. Patients with positive results may not have clinical signs and symptoms; removing the adult worms surgically from these patients will prevent the occurrence of lymphatic filariasis.
Lymphoscintigraphy is the single most useful tool in establishing the diagnosis, grading, and cause (see Chapter 26). It can show the outcome of treatment after chemotherapy or surgery.
MRI is useful in the presence of associated problems. MR lymphangiography is a very good assessment modality but very expensive in a country such as India, where health coverage by insurance or the government is not available (see Chapter 28).
Assessment
Lymphedema can be objectively assessed by several methods, ranging from clinical evaluation to radiologic modalities. One clinical method involves measuring the limb circumference at various points. The upper limb is divided into four segments. The upper limit of measurement, known as the 65% point, is marked on the upper arm 65% of the distance from the olecranon to the acromion tip. The four segments are (1) the wrist at the level of ulnar styloid to the midforearm, (2) the midforearm to the elbow at the level of olecranon, (3) the elbow to the midarm, and (4) the midarm to the 65% point. These segments conform to the shape of a truncated cone. Measurements obtained using this method produce the least standard error of measurement. The volume of each segment can be calculated using the following formula:
Segment volume = h(C12 + C22 + [C1 × C2])/12π
where h is the length of each segment and C1 and C2 are the circumference of each segment at both ends. The sum of the segment volumes is the volume of the limb. 8
Another clinical method is water displacement volumetry. The patient’s upper limb is immersed in a graduated steel cylinder up to the 65% point. The volume of water displaced can be calculated using the following formula:
Volume = πr2h
where r is the radius of the cylinder and h is the height of the water displaced.
Calculation of the volume of edema in unilateral cases is recommended. This is done by estimating the difference in the limb volume between the edematous limb and the normal limb.
In lymphedema patients, high-frequency sonography is useful for assessing the thickness of the skin and the subcutaneous tissue. The patient can be sitting or lying down, with the limb extended to allow access to all aspects—anterior, posterior, medial, and lateral—to determine the average value. Compressibility of the regional veins can be assessed to rule out venous thrombosis.
Lymphoscintigraphy using technetium sulphur colloid is a useful modality for studying the lymphatics preoperatively and postoperatively. It reliably allows visualization of the lymphatic vessels and lymph flow and is helpful in distinguishing between primary and secondary lymphedema. 9 It is performed after intradermal injection of Tc99m (technetium) colloid in the web spaces of the lower limbs (see Chapter 26).
Medical Management
The treatment options for patients with lymphedema are broadly divided into two categories: medical and surgical. Many methods used in the past, such as crepe bandaging and flap transfers, are now rarely used. The following recommendations apply to all four grades of lymphedema:
Foot care
Avoidance of injuries and injections to the affected limb
Elimination of the focus of sepsis
Complete decongestive therapy with bandaging, followed by placement of pressure garments
Complete decongestive therapy is an important modality in the management of lymphedema. It consists of manual manipulation of the lymphatic ducts, short stretch compression bandaging, therapeutic exercises, and meticulous skin care.
Elevation of the affected part
Management of acute attacks
Cyclic chemotherapy (antibiotic and antifilarial) to prevent secondary infection
MAINTENANCE OF LIMB HYGIENE
Filarial patients with damaged lymphatic vessels often have more bacteria on the skin than those without this disease; hence infections are very common. The large number of bacteria on the skin, multiple skin lesions, slow lymph fluid movement, and the reduced ability of the lymph nodes to filter bacteria cause inflammation characteristic of an acute attack. Washing the limb is essential to block this vicious cycle. Clean water at room temperature should be used. The least expensive soap without perfume is usually best. After the area is washed, it should be gently towel dried. This should be done twice daily, ideally in the morning and at night.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree