Chancroid: Introduction
|
Epidemiology
Chancroid is most common in developing countries, especially in Africa and Asia, where it was isolated from over 50% of patients with genital ulcers until the 1990s.1–3 These endemic regions also have some of the highest rates of HIV infection in the world, and chancroid is common in all 18 countries where adult HIV prevalence surpasses 8%.4 More recent reports from Southeast Asia and Africa suggest that the incidence of chancroid may be declining in the face of a rapidly rising incidence of genital herpes.5–9 Chancroid outbreaks have been reported in a number of cities in industrialized countries during the last two decades, predominantly in the United States.10 After an epidemic in California in 1981, the number of cases peaked in 1987 at 5,035 cases. In a ten city study, chancroid was confirmed in 12% of genital ulcers in Chicago and 20% in Memphis.11 In contrast, only 23 cases of chancroid were reported to the Centers for Disease Control and Prevention (CDC) in 2007.12 The true incidence in most areas remains unclear and is probably vastly underreported because confirmatory culture media or DNA amplification methods are not commercially available.13 The global epidemiology of chancroid is so poorly documented that it is not included in WHO estimates of the global incidence of curable sexually transmitted diseases.4 Overall, chancroid accounted for 8 cases (3%) of genital ulcers in a sexually transmitted infection (STI) clinic in Paris from 1995 to 2005.14
The prevalence of chancroid is higher in lower socioeconomic groups. Recent epidemics in the industrialized countries have usually been associated with commercial sex work, the use of crack cocaine, with syphilis and an increased risk of HIV infection.15,16 Lower-class prostitutes appear to be a reservoir in all reported outbreaks of this disease and men have a markedly higher incidence of chancroid than women.10 Several studies in Africa showed that chancroid-ulcer is an important risk factor for the heterosexual spread of HIV-1.17,18 In West Africa, it has been shown that 2% of female sex workers were carrying the organism asymptomatically.19 The duration of infectivity in the absence of treatment was estimated to be 45 days for women. The transmission rate from females to males is not known, in contrast to a reported transmission rate from males to females of 70% per sex act.20 Nonsexual transmission has been recently reported.21,22
Etiology and Pathogenesis
![]() Chancroid, or soft chancre (ulcus molle), was first distinguished from syphilis by Basserau in France in 1842. The causative bacillus was discovered and described by Ducrey in 1889, a bacteriologist at the University of Naples. Unna described the histology of the chancroidal ulcer and found the chains of Gram-negative rods in the lesion. It is still unclear who was the first to culture Haemophilus ducreyi. In his review, Albritton23 credited Himmel (1901) with the first convincing isolation of H. ducreyi, but other authors in the same period also claimed priority.
Chancroid, or soft chancre (ulcus molle), was first distinguished from syphilis by Basserau in France in 1842. The causative bacillus was discovered and described by Ducrey in 1889, a bacteriologist at the University of Naples. Unna described the histology of the chancroidal ulcer and found the chains of Gram-negative rods in the lesion. It is still unclear who was the first to culture Haemophilus ducreyi. In his review, Albritton23 credited Himmel (1901) with the first convincing isolation of H. ducreyi, but other authors in the same period also claimed priority.
![]() H. ducreyi is a Gram-negative, facultative anaerobic coccobacillus that requires hemin (X factor) for growth. The organism is small, nonmotile, and nonspore-forming and shows typically streptobacillary chaining, especially in culture. The exact taxonomy is still controversial. The current classifications list H. ducreyi as a true Haemophilus species. However, studies of DNA homology and chemotaxonomy demonstrate substantial differences between H. ducreyi and Haemophilus species. H. ducreyi will likely be reclassified in the future, but this issue awaits further studies.23,24
H. ducreyi is a Gram-negative, facultative anaerobic coccobacillus that requires hemin (X factor) for growth. The organism is small, nonmotile, and nonspore-forming and shows typically streptobacillary chaining, especially in culture. The exact taxonomy is still controversial. The current classifications list H. ducreyi as a true Haemophilus species. However, studies of DNA homology and chemotaxonomy demonstrate substantial differences between H. ducreyi and Haemophilus species. H. ducreyi will likely be reclassified in the future, but this issue awaits further studies.23,24
![]() H. ducreyi has few distinguishing biochemical features. Nitrate reduction is a characteristic of the genus. All reported strains are oxidase-positive and catalase-negative and have a broad range of phosphatase activity. The alkaline phosphatase reaction is used in its identification.23 Differentiation from other hemin-requiring strains of Haemophilus is made by the lack of requirement for nicotinamide adenine dinucleotide (NAD, V factor) and its failure to produce hydrogen sulfide, catalase, or indole.23,25
H. ducreyi has few distinguishing biochemical features. Nitrate reduction is a characteristic of the genus. All reported strains are oxidase-positive and catalase-negative and have a broad range of phosphatase activity. The alkaline phosphatase reaction is used in its identification.23 Differentiation from other hemin-requiring strains of Haemophilus is made by the lack of requirement for nicotinamide adenine dinucleotide (NAD, V factor) and its failure to produce hydrogen sulfide, catalase, or indole.23,25
![]() H. ducreyi is a fastidious bacillus. In order to get optimal rates of positive cultures, Nszane et al26 recommend the use of two media simultaneously: (1) gonococcal agar supplemented with bovine hemoglobin and (2) Mueller-Hinton agar supplemented with chocolate horse blood, each with 5% fetal calf serum and vancomycin. Growth is best at 30°C–33°C (86°F–91.4°F) in a water-saturated atmosphere.
H. ducreyi is a fastidious bacillus. In order to get optimal rates of positive cultures, Nszane et al26 recommend the use of two media simultaneously: (1) gonococcal agar supplemented with bovine hemoglobin and (2) Mueller-Hinton agar supplemented with chocolate horse blood, each with 5% fetal calf serum and vancomycin. Growth is best at 30°C–33°C (86°F–91.4°F) in a water-saturated atmosphere.
![]() The H. ducreyi genome is being cloned. H. ducreyi shares a significant gene pool with members of the Pasteurellaceae and the Enterobacteriae. The core plasmid conferring ampicillin resistance in H. ducreyi is found in other species of Haemophilus and Neisseria.23 β-Lactamase production is mediated by plasmids (5,000 and 5,700 kDa) identical to the two types of Neisseria gonorrhoeae β-lactamase plasmid. A 3,000- and 3,200-kDa plasmid mediating β-lactamase production has also been reported.27 The reassortment of the different plasmids in various isolates can be used as a partial typing system. Isogenic mutants of H. ducreyi now can be constructed and the virulence properties of specific mutants can be determined. An isogenic hemoglobin receptor-deficient mutant of H. ducreyi was reported that showed an attenuated infection in a human model.28
The H. ducreyi genome is being cloned. H. ducreyi shares a significant gene pool with members of the Pasteurellaceae and the Enterobacteriae. The core plasmid conferring ampicillin resistance in H. ducreyi is found in other species of Haemophilus and Neisseria.23 β-Lactamase production is mediated by plasmids (5,000 and 5,700 kDa) identical to the two types of Neisseria gonorrhoeae β-lactamase plasmid. A 3,000- and 3,200-kDa plasmid mediating β-lactamase production has also been reported.27 The reassortment of the different plasmids in various isolates can be used as a partial typing system. Isogenic mutants of H. ducreyi now can be constructed and the virulence properties of specific mutants can be determined. An isogenic hemoglobin receptor-deficient mutant of H. ducreyi was reported that showed an attenuated infection in a human model.28
![]() Three major problems seem to be important in the still incompletely understood pathogenesis of H. ducreyi infection: (1) the adherence to the epithelial surface, (2) the rate of exotoxins (e.g., cytolethal distending toxin),29 and (3) the resistance to the host defense mechanism. Various other virulence factors (e.g., fibrinogen-binding lipoprotein and other binding proteins) are described.30,31 Many questions about pathogenesis are still unclear, but a human model of H. ducreyi infection has provided more detailed experimental analyses of the interactions between humans and H. ducreyi than is available for most bacterial infections.32
Three major problems seem to be important in the still incompletely understood pathogenesis of H. ducreyi infection: (1) the adherence to the epithelial surface, (2) the rate of exotoxins (e.g., cytolethal distending toxin),29 and (3) the resistance to the host defense mechanism. Various other virulence factors (e.g., fibrinogen-binding lipoprotein and other binding proteins) are described.30,31 Many questions about pathogenesis are still unclear, but a human model of H. ducreyi infection has provided more detailed experimental analyses of the interactions between humans and H. ducreyi than is available for most bacterial infections.32
Clinical Findings
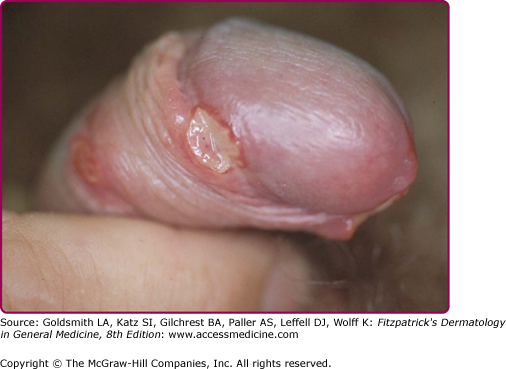
The incubation period is between 3 and 7 days, rarely more than 10 days. No prodromal symptoms are known. The chancre begins as a soft papule surrounded by erythema. After 24–48 hours it becomes pustular, then eroded and ulcerated (Fig. 202-1); vesicles are not seen. The edges of the ulcers are often ragged and undermined (Fig. 202-2). The ulcer is usually covered by a necrotic, yellowish-gray exudate (eFig. 202-2.1), and its base is composed of granulation tissue that bleeds readily on manipulation. In contrast to syphilis, chancroid ulcers are usually tender and or painful not indurated (soft chancre). The diameter varies from 1 mm to 2 cm. Half of the males present with a single ulcer and most lesions are found on the external or internal surface of the prepuce, on the frenulum, or on the glans (eFig. 202-2.2). Meatus and shaft of the penis and the anus (eFig. 202-2.3) are involved less frequently. Edema of the prepuce is often seen. Rarely, if the chancre is localized in the urethra, Haemophilus ducreyi causes purulent urethritis.33
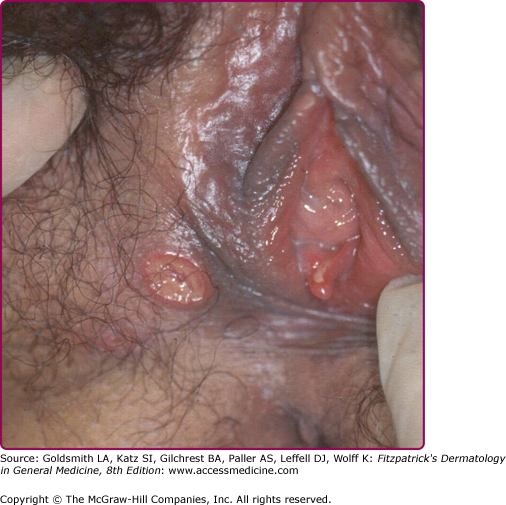
In females the lesions are mostly localized on the vulva (Fig. 202-3), especially on the fourchette, the labia minora, and the vestibule. Vaginal, cervical, and perianal ulcers have also been described. Extragenital lesions of chancroid have been reported on the breasts, fingers, thighs, and inside the mouth. Trauma and abrasion may be important for such extragenital manifestations.
Painful inguinal adenitis (bubo) occurs in up to 50% of patients within a few days to 2 weeks (average 1 week) after onset of the primary lesion (Fig. 202-4). The adenitis is unilateral in most patients, and erythema of the overlying skin is typical. Buboes can become fluctuant and may rupture spontaneously. The pus of bubo is usually thick and creamy. Buboes are less common in female patients. Besides the common types of chancroid described above, a number of clinical variants have been reported (Table 202-1). Mild systemic symptoms can rarely accompany chancroid, but systemic infection by H. ducreyi has never been observed. The significance of the recent detection of genetic material of H. ducreyi in oesophageal lesions of HIV patients34 requires further study.
Giant Chancroid | Single lesion extends peripherically and shows extensive ulceration. |
Large Serpiginous Ulcer | Lesion that becomes confluent, spreading by extension and autoinoculation. The groin or thigh may be involved (Ulcus molle serpiginosum). |
Phagedaenic Chancroid | Variant caused by superinfection with fusospirochetes. Rapid and profound destruction of tissue can occur (Ulcus molle gangraenosum). |
Transient Chancroid | Small ulcer that resolves spontaneously in a few days may be followed 2–3 weeks later by acute regional lymphadenitis (French: chancre mou volant). |
Follicular Chancroid | Multiple small ulcers in a follicular distribution. |
Papular Chancroid | Granulomatous ulcerated papule may resemble donovanosis or condylomata lata (Ulcus molle elevatum). |
Laboratory Tests
![]() Bacterial culture of H. ducreyi currently remains the main tool for the diagnosis of chancroid in the clinical setting. However, the advent of more sensitive DNA amplification techniques has demonstrated that the sensitivity of culture of H. ducreyi reaches only 75% at the best.35–37 The bacillus will survive only 2–4 hours on a swab unless refrigerated. Swabs from the purulent ulcer base should be inoculated directly to appropriate culture medium since no satisfactory transport system is available.38
Bacterial culture of H. ducreyi currently remains the main tool for the diagnosis of chancroid in the clinical setting. However, the advent of more sensitive DNA amplification techniques has demonstrated that the sensitivity of culture of H. ducreyi reaches only 75% at the best.35–37 The bacillus will survive only 2–4 hours on a swab unless refrigerated. Swabs from the purulent ulcer base should be inoculated directly to appropriate culture medium since no satisfactory transport system is available.38
![]() The simultaneous use of two primary isolation media from a nutritionally rich agar base supplemented with hemoglobin and serum are recommended for high culture sensitivity.26 Small, nonmucoid, yellow–gray, translucent colonies appear in 2–4 days after inoculation. Typically, these colonies remain intact when they are pushed across the agar surface. The identification of H. ducreyi
The simultaneous use of two primary isolation media from a nutritionally rich agar base supplemented with hemoglobin and serum are recommended for high culture sensitivity.26 Small, nonmucoid, yellow–gray, translucent colonies appear in 2–4 days after inoculation. Typically, these colonies remain intact when they are pushed across the agar surface. The identification of H. ducreyi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree