17 Carcinoma of the upper aerodigestive tract
Synopsis
 The incidence of head and neck cancer in the US is declining Head and neck squamous cell cancers (HNSCCs) as a group are the fifth most common malignancy among men worldwide.
The incidence of head and neck cancer in the US is declining Head and neck squamous cell cancers (HNSCCs) as a group are the fifth most common malignancy among men worldwide.
 There is an association between some HNSCCs and human papillomavirus (HPV). Human papilloma virus-related HNSCC is reaching epidemic proportions in the US and will eclipse the incidence of cervical cancer over the forthcoming years.
There is an association between some HNSCCs and human papillomavirus (HPV). Human papilloma virus-related HNSCC is reaching epidemic proportions in the US and will eclipse the incidence of cervical cancer over the forthcoming years.
 Cigarette smoking and chewing carcinogenic stimulants are a significant etiologic factor worldwide.
Cigarette smoking and chewing carcinogenic stimulants are a significant etiologic factor worldwide.
 Alcohol, too, is an important promoter of carcinogenesis and is a contributive factor in at least 75% of HNSCCs.
Alcohol, too, is an important promoter of carcinogenesis and is a contributive factor in at least 75% of HNSCCs.
 Diagnosis is made by history, physical examination, and appropriate imaging.
Diagnosis is made by history, physical examination, and appropriate imaging.
 Any adult patient who presents with complaints of a neck mass should be considered a malignancy until proven otherwise.
Any adult patient who presents with complaints of a neck mass should be considered a malignancy until proven otherwise.
 There is an emerging role for robotic surgery of the upper aerodigestive tracts in the management of HNSCC.
There is an emerging role for robotic surgery of the upper aerodigestive tracts in the management of HNSCC.
Introduction
Approximately 48 000 new cases of head and neck cancer were projected to be diagnosed in the US, with over 11 000 Americans succumbing to these malignancies in 2008.1 Head and neck cancer, predominantly SCC, accounts for only 3% of all new cancer cases and only 2% of all cancer deaths in the US annually; however, these malignancies as a group are the fifth most common malignancy among men worldwide.2 The overall incidence in the US appears to be decreasing in parallel with the increased awareness of the effects of cigarettes and smoking on the development of upper aerodigestive cancer; however, head and neck cancers continue to plague many parts of the world, especially where cigarette smoking and/or the chewing of carcinogenic stimulants is prevalent, and thus head and neck cancers will continue to be a major cause of cancer mortality worldwide.2,3 A rising proportion of these cancers (particularly those found in the oropharynx) are attributable to oncogenic HPV4,5 and the impact that the population-wide HPV vaccination will have on incidence rates has yet to be determined. This remains the most rapidly increasing segment of head and neck cancer incidence in the US today. Evidence is also suggestive that inherited factors and exposure to other environmental agents such as chromium, nickel, wood dust, industrial agents, and formaldehyde modulate risk and awareness will help refine prevention strategies in the future.3,6,7
This chapter aims to describe cancers of the upper aerodigestive tract in an anatomical fashion, but is not meant to be exhaustive. We will review the relevant anatomy, the American Joint Committee on Cancer staging criteria, and important treatment algorithms and considerations. Salivary gland tumors are addressed in a separate section (Chapter 14). It is the purpose of this chapter to summarize the surgical principles appropriate for all patients undergoing for carcinoma of the upper aerodigestive tract. Specific reconstructive modalities will be covered in subsequent chapters.
Incidence and prevalence
In the US, estimates for 2012 were for 26 740 new cases of oral cavity cancer, 13 510 new cases of pharyngeal cancer, and 12 360 new cases of laryngeal cancer.4 While the US has noted an initial decrease in the incidence of head and neck malignancies, current studies have noted a recent increase in incidence.5–8 In particular, while the median age at diagnosis for HNSCC is approximately 60 years, the incidence in patients younger than 45 years old appears to be increasing, presumably secondary to oncogenic strains of HPV.9,10 Regarding cancer survivorship, approximately 350 000 individuals were living in the US with a history of head and neck cancer in November, 2007 (240 176 with a history of oral cavity/pharyngeal cancer and 93 096 with a history of laryngeal cancer). In 2008 in the US, 5390 deaths were predicted to be attributed to oral cavity cancer, 2200 to pharyngeal cancer, and 3670 to laryngeal cancer. However, head and neck cancer accounts for less than 2% of all cancer deaths in the US annually, and mortality rates appear to be decreasing.
Risk factors
Tobacco
The strength and consistency of the association between smoking and HNSCC have been demonstrated in numerous case-control and cohort studies with significant relative risks or odds ratios in the 3–12-fold range.11–16 In addition, these follow-up studies have demonstrated a dose-dependent effect where higher pack years of smoking (packs per day × years of smoking) correlated with an increased risk while a longer duration since smoking cessation was associated with a decreased risk of developing a malignancy.12,13,15,17 While there is a definitive relationship between tobacco use and upper aerodigestive SCC, the association with other mucosal malignancies such as nasopharyngeal and nasosinus carcinomas is less substantial.18 The etiological relationship encompasses cigar and pipe smoking as well; however, the risk of second-hand smoke is less clear.16,17,19,20 Regarding tobacco consumption and usage aside from smoking, there appears to be a strong correlation with the location of chewless tobacco or tobacco equivalents and the location of resultant malignancies. For example, such patients often develop SCC in dependent areas of the oral cavity such as the floor of mouth, larynx, and hypopharynx.21 Similarly, in south central Asia where the use of such products is common, the gingivobuccal region is the most common site for HNSCC.3,22 Furthermore, in South Central Asia “pano” (betel leaf, lime, catechu, and areca nut) is commonly chewed and is a strong risk factor independent of tobacco use for carcinoma of the oral cavity, one of the most common cancers in men and women in this region.3,23,24
Alcohol
Alcohol, too, is an important promoter of carcinogenesis and is a contributive factor in at least 75% of HNSCCs.12,15,17 Furthermore, alcohol appears to have an effect on risk of HNSCC independent of tobacco smoking, but these effects are consistently significant only at the highest level of alcohol consumption.6,12,15,16 While there is speculation as to the exact type of alcohol and the development of head and neck malignancies, it appears that the causative agent is the ethanol itself and the quantity consumed, regardless of the form in which it is consumed (i.e., beer, wine).15,25 Nevertheless, it appears that the major clinical significance of alcohol consumption is that it potentiates the carcinogenic effect of tobacco at every level of tobacco use. However, this effect is most striking at the highest levels of exposure. The magnitude of this effect is at least additive, but may be synergistic in its carcinogenic effects.12,15
Infectious agents
Although it has been suggested that various infectious agents play a role in head and neck carcinomas, only EBV and HPV can be implicated as etiologic agents in head and neck carcinogenesis based on current scientific evidence. EBV appears to be associated with most nasopharyngeal carcinomas, and HPV (most commonly type 16 and 18) is associated with approximately 50% of oropharyngeal carcinomas.5,6 HPV-related HNSCC appears to be a distinct biologic entity, with an excellent prognosis and exquisite chemo-radiosensititivty. HPV may also play a role in the etiology of SCC arising in the sinonasal tract.26 While other infectious agents such as herpes simplex viruses and Helicobacter pylori have not been purported to be carcinogenic, no definitive evidence has been shown to support a causal relationship.27–30 On the contrary, oropharyngeal cancer patients presenting without an extensive smoking history or tobacco exposures more commonly have HPV-16-associated tumors,21 which may also suggest that there are synergistic interactions between the traditional oropharyngeal risk factors of tobacco and alcohol with HPV-16.31 However, this is an area of continued research and investigation and brings into question whether the potential benefits of HPV-16 vaccination in preventing cervical cancer may extend to the prevention of upper aerodigestive malignancies as well.10
Laryngopharyngeal reflux
There is currently an active debate regarding the contribution of gastroesophageal reflux and consequent exposure of the upper aerodigestive tract to gastric contents and bacteria (i.e., H. pylori) in relation to the incidence of head and neck malignancies. Observational and anecdotal studies have long suggested that gastroesophageal reflux documented with 24-hour pH probe monitoring may be associated with laryngeal cancer.32–37 Furthermore, a retrospective case-control study of 10 140 hospitalized patients and 12 061 outpatients with laryngeal and pharyngeal cancer and 40 561 hospitalized and 48 244 outpatient controls performed using US Department of Veterans Affairs databases demonstrated a significant increased risk of developing an upper aerodigestive cancer (laryngeal cancer).38 However, a large Swedish cohort study of 66 965 patients with discharge diagnoses of heartburn, hiatal hernia, or esophagitis with a follow-up of 376 622 person-years concluded that there was no evidence of a causal association between gastroesophageal reflux and either laryngeal or pharyngeal cancer.39
Occupation/air pollution
Although occupational exposures probably play a minor role overall in the development of HNSCC, they are major risk factors for malignancies of the sinonasal region.40–44 The most important exposures occur in the metalworking, refining, woodworking, and leather/textile industries.40–43 Indoor air pollution is a significant problem in much of the developing world where indoor stoves using biomass or fossil fuels are the primary method of cooking and heating. Not only are these exposures likely risk factors for HNSCC, but they also contribute to the risk of paranasal sinus cancers and lung cancers, chronic pulmonary diseases, and childhood illnesses.
Anatomy
Oral cavity
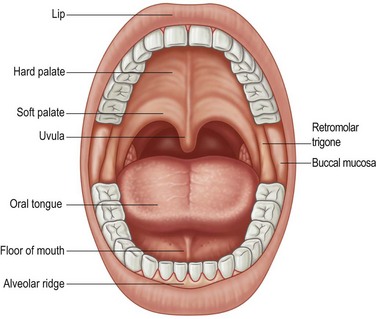
The oral cavity is defined as starting at the vermilion border of the lips and extends posteriorly to include the buccal mucosa, anterior tongue, and floor of the mouth, hard palate, and upper and lower gingiva (Fig. 17.1). The posterior border is defined by the circumvallate papillae of the tongue. Consequently, the mobile tongue occupies a major portion of the oral cavity and is contiguous with the floor of the mouth. The gingival mucosa overlying the mandibular and maxillary alveolar ridges adheres to the underlying periosteum. The hard palate forms the roof of the oral cavity and consists of mucosa overlying the palatine portion of the maxilla extending from the superior alveolar ridge to the junction with the soft palate, which lies in the oropharynx. Although the delineation between oral cavity and oropharynx might seem artificial, the distinction is important because of varying risk factors, natural history, individualized therapeutic approaches, and numerous functional considerations.
Pharynx
The pharynx is a musculomembranous tube suspended from the skull base to the level of the sixth cervical vertebra, supported by overlapping constrictor muscles (superior, middle, and inferior) and other muscles arising from the styloid process and skull base (Fig. 17.2). This musculomembranous conduit communicates with the oral cavity anteriorly, the nasopharynx superiorly, and the hypopharynx and larynx inferiorly. The nasopharynx is bounded by the skull base and represents the cranial-most segment of the pharynx and includes the soft palate, the adenoids, and superior portion of the pharyngeal walls, which contain the opening of the eustachian tubes.
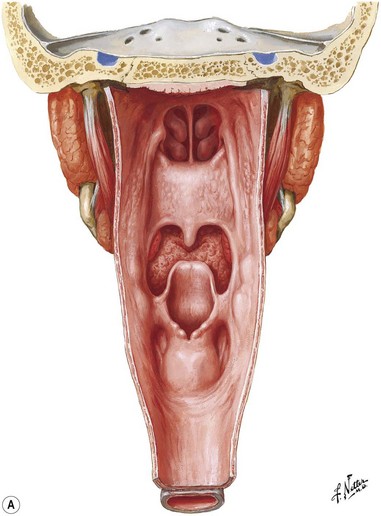
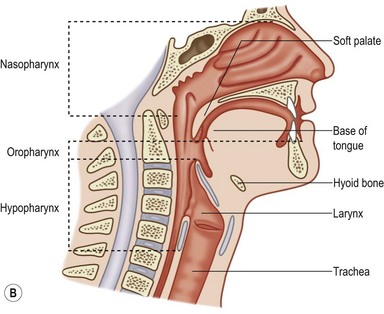
Fig. 17.2 Pharynx. (A) Posterior view; (B) sagittal view.
(Netter illustration from www.netterimages.com copyright Elsevier Inc. All rights reserved.)
Larynx
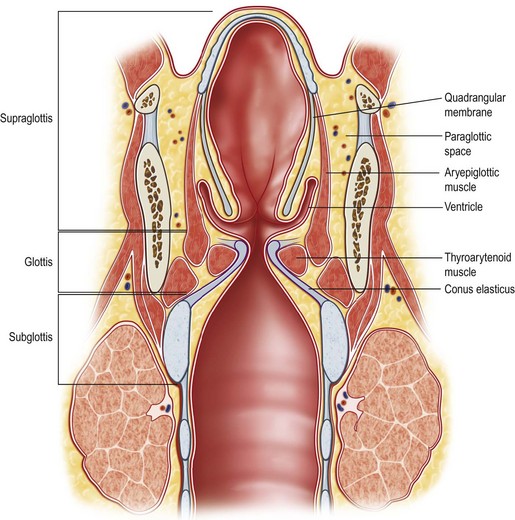
The larynx consists of a mucosally covered cartilaginous framework (thyroid and cricoid cartilages) suspended from the hyoid bone above by the thyrohyoid membrane and attached below to the trachea (Fig. 17.3). The opening to the larynx is continuous with the pharyngeal airway. Unlike the rest of the pharynx, the mucosa of the larynx consists largely of columnar, ciliated, respiratory-type epithelium. Stratified squamous epithelium is found on the upper posterior epiglottis, aryepiglottic folds, and true vocal folds. It is important to note that, although there are lymphatics in the upper larynx, they are sparse in the true vocal folds, or glottis. The larynx is divided into three anatomic regions: the supraglottic larynx, the glottic larynx, and the subglottic larynx. The supraglottic larynx includes the epiglottis, aryepiglottic folds, and laryngeal surface of the arytenoids, false vocal cords, and ventricles. The glottic larynx is derived from the tracheobronchial anlage and consists of both true vocal cords and the mucosa of the anterior and posterior commissures. It extends from the lateral-most apex of the laryngeal ventricle to 1 cm below the free edge of the vocal folds toward the cricoid. It has few, if any, lymphatics. The subglottic larynx consists of the region bounded by the glottis above and the inferior border of the cricoid cartilage. Lymphatic supply to the subglottic larynx is extensive and bilateral. The infraglottic lymphatics drain to the cervical nodes through the cricothyroid membrane, while supraglottic lymphatics drain through the thyrohyoid membrane.
Nose and paranasal sinuses
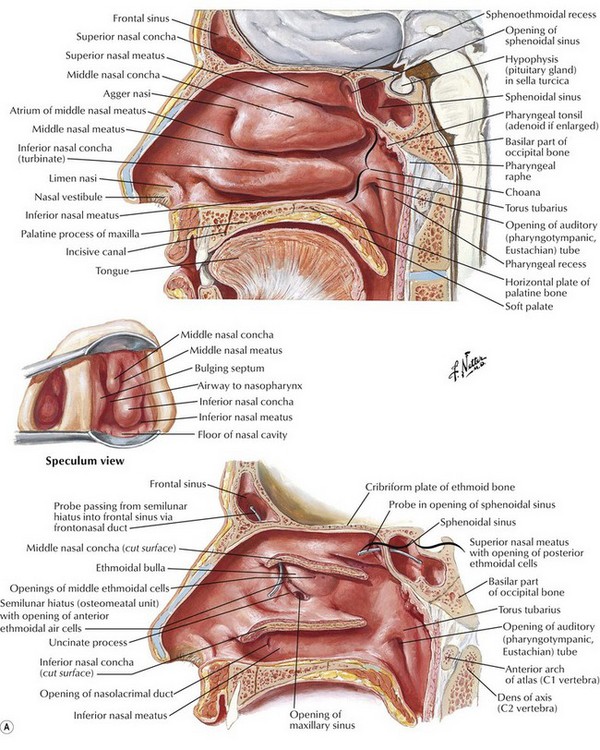
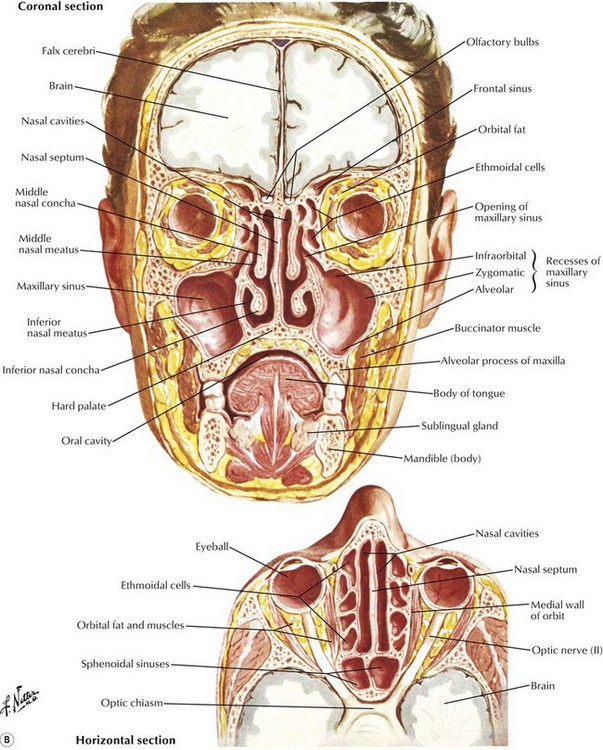
The term “nose and paranasal sinuses” refers to the region of the upper aerodigestive tract that starts at the vestibule of the nose anteriorly, is covered by squamous epithelium, and extends posteriorly to the posterior choana, where the nasopharynx begins. The nasal cavity begins at the nostrils and ends at the nasal choanae, which communicate with the nasopharynx and include the vestibules, turbinates, septum, and choanae (Fig. 17.4). By definition, paranasal sinus malignancy does not include the nasopharynx unless by extension. It does include the paranasal sinuses, specifically, the maxillary, ethmoid, frontal, and sphenoid sinuses. Although the most common malignancy of the nose and paranasal sinuses is SCC, the nose and paranasal sinuses pose a particular set of problems that deserve separate consideration.
Neck
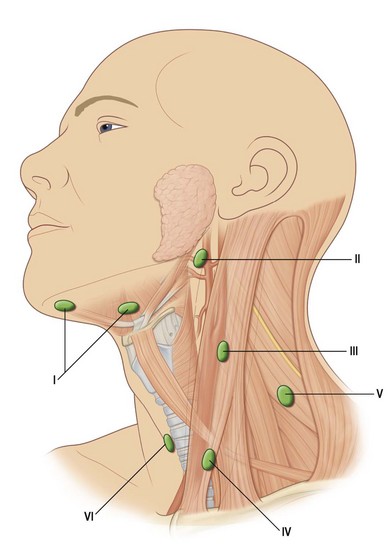
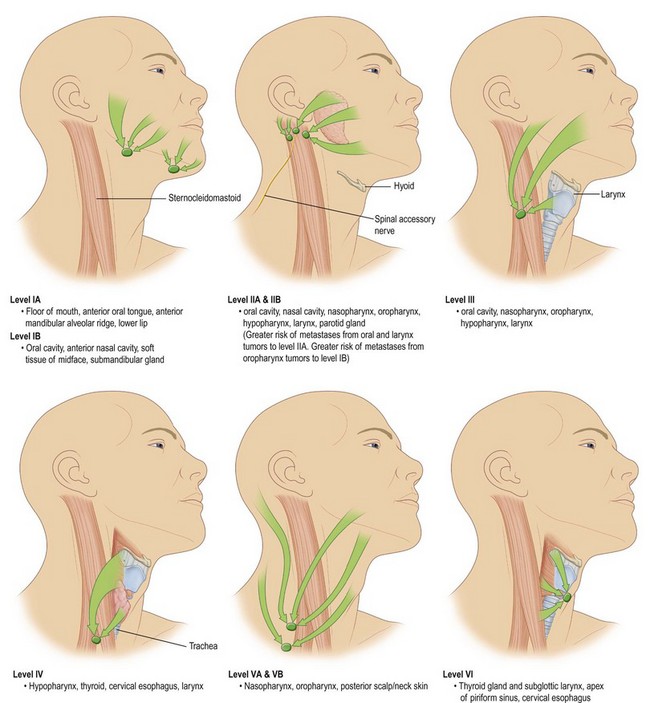
Anatomic considerations in the treatment of cancers of the head and neck must include a thorough understanding of the neural, vascular, and, especially, the lymphatic structures of the neck. Specifically, the digastric, omohyoid, sternocleidomastoid, and trapezius muscle all help contribute to organizing the neck into specific anatomical regions. These specific regions of the head and neck and the tumors that arise there have lymphatic drainage that have traditionally been thought of as being consistent and predictable. However, the use of sentinel lymph node biopsy in the management of HNSCCs would indicate that this may not be valid. Currently, the role of sentinel lymph node biopsy in the management of HNSCC is being evaluated.45 There are six major groups of lymph nodes (paired bilaterally) in the head and neck,46 although only levels I–V play a major role in aerodigestive tract HNSCC (Fig. 17.5).
Primary and secondary echelons of lymph node drainage have been defined for each major region of the head and neck mucosa. A standard rule of thumb is that the lymphatic drainage for any particular region is predicted by the arterial supply of that region. The lip, cheek, and anterior gingiva drain to submandibular and submental lymph node groups. In addition, the cheek and upper lip also drain to inferior parotid and facial nodes, while the posterior gingiva and palate drain to the internal jugular chain and lateral retropharyngeal groups. Lymphatic drainage for the tongue drains to the internal jugular, subdigastric, omohyoid, submandibular, and submental nodal groups. Midline lesions often drain bilaterally. The floor-of-mouth drainage is similar to that of the tongue. The upper portion of the pharynx drains directly to the upper cervical lymph nodes along the internal jugular chain. The oropharynx and tonsil drain through the parapharyngeal space to the midjugular region, particularly to the jugulodigastric nodes. Retropharyngeal nodes (nodes of Rouvière) and lateral pharyngeal nodes can also be involved and are always pathologic when clinically apparent. The regions of the hypopharynx and larynx drain primarily along the routes of their vascular supply to either the deep cervical nodes along the mid jugular (upper pharynx, larynx) or the deep nodes along the lower jugular and paratracheal region (lower pharynx, larynx) (Fig. 17.6).
Diagnosis and treatment
Oral cavity
The oral cavity begins at the lips and extends posteriorly to the circumvallate papillae and therefore is the area most accessible not only to direct examination, but also the area subjected to sun and ultraviolet exposure and resultant SCC of the lips. Because of the accessibility of the oral cavity, and the morbidity of radiation-induced xerostomia, early tumors of this area (T1 and T2) are generally treated surgically. Locally advanced lesions (T3 and T4) are typically treated with combination therapy. While pre-malignant and small tumors of the lips can sometimes be treated with radiation or topical agents such as 5-fluorouracil or imiquimod, the standard treatment for lip cancers is surgery. Malignancies of the lip tend to be SCC while malignancies of the skin around the lip tend to be basal cell carcinomas. All lip cancers mandate assessment of the neck, but larger lip lesions warrant evaluation for possible selective neck dissection. Neck dissections are typically accomplished through an apron or visor-type incision and are often performed for advanced-stage lesions, although simultaneous neck and lip surgery may have implications for reconstructive options (Fig. 17.7). Synchronous neck dissections for lip cancers will impact reconstructive options, and the low incidence of occult cervical metastasis has led to a policy of neck observation for the majority of patients with lip cancers. Upper lip and commissure malignancies may drain to periparotid nodes, which require evaluation and possible superficial parotidectomy, in selected cases. Reconstruction of the lips will be discussed elsewhere (Chapter 10).
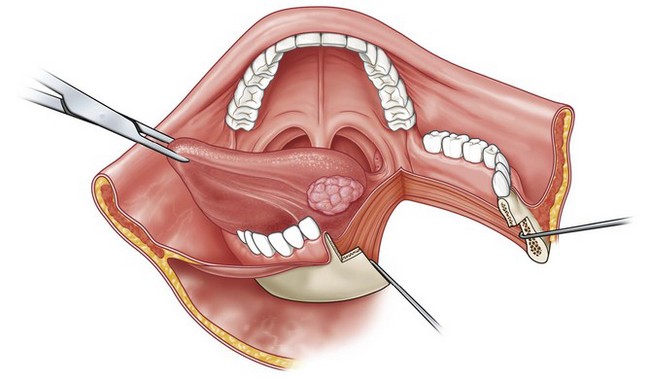
Fig. 17.7 Mandible-splitting approach for access to posterior tongue tumors.
(Reproduced from from Guyuron B, Erikkson E, Persing J, et al. Plastic surgery: indications and practice. Philadelphia, PA: Elsevier; 2008.)
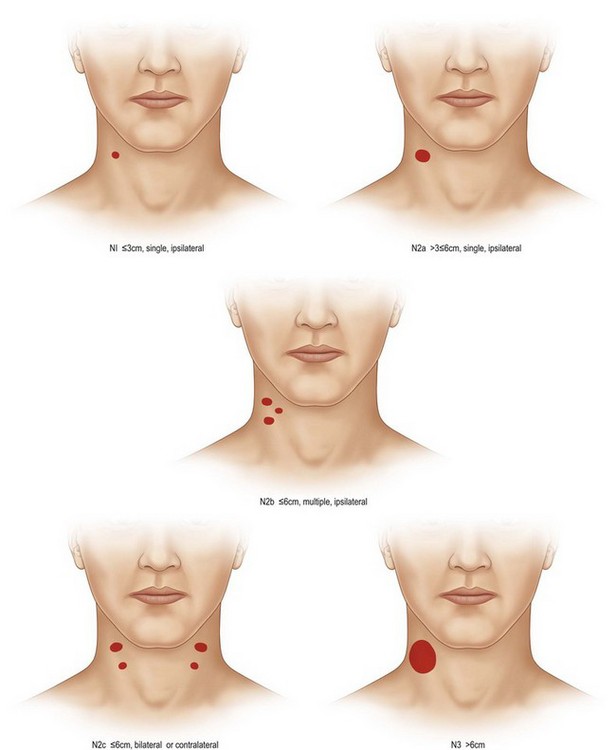
All tumors of the lip and oral cavity are staged using the tumor, node, and metastasis (TNM) classification (Table 17.1 and Fig. 17.8). Postoperative irradiation is often indicated for oral cancers, which can pose challenges for reconstruction, dentition, and rehabilitation. The indications for adjuvant radiation in oral cancer include: close or positive margins, perineural invasion, multinodal metastasis, bony or soft-tissue invasion, and T3 or T4 tumors. While the lips comprise approximately 30% of cancers of the oral cavity, approximately 25–50% of oral cavity cancers occur in the mobile tongue. Because of the lack of anatomic barriers to spread, tongue cancer has a propensity for diffuse, infiltrative involvement, which is often difficult to gauge clinically. Curative resection, therefore, mandates an adequate cuff (generally 1 cm) around the lesion. T1 or T2 lesions are usually amenable to transverse wedge excision and subsequent direct closure or skin grafting. Large T2 lesions and larger lesions may require a paramedian mandibulotomy in order to obtain exposure for resection and reconstruction, which often requires free tissue transfer (Fig. 17.9). Floor-of-mouth cancers and cancers of the alveolar ridge comprise 30% of oral cavity malignancies and are intimately associated with the dentition and the mandible. If the cancer invades the bone, it may extend along the alveolar canal. Tumors of the retromolar trigone and buccal mucosa are often difficult to treat given their location and may require a mandibulectomy to gain appropriate access, exposure, and less common oncological control. Finally, tumors of the hard palate are uncommon and tend to originate from the minor salivary glands, which will be discussed later in this chapter. Depending on tumor size and location, resection may be performed perorally, through a transoral midface degloving approach, or through a Weber–Ferguson approach. Reconstruction of the hard palate (to maintain speech, feeding, and the separation of the oral cavity from the nasal cavity), nasal lining, and orbital floor may be required. Due to the high frequency of occult nodal metastasis, elective neck dissection is performed at the time of surgical resection. Clinical nodal disease mandates a therapeutic neck dissection.
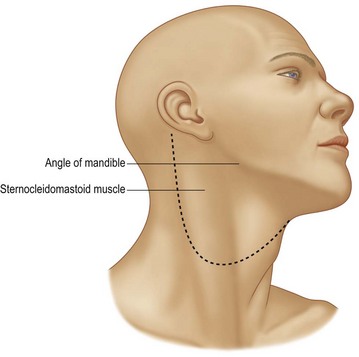
Fig. 17.8 Apron incision for access when performing neck dissections.
(Reproduced from from Guyuron B, Erikkson E, Persing J, et al. Plastic surgery: indications and practice. Philadelphia, PA: Elsevier; 2008.)
Table 17.1 TNM staging of the lip and oral cavity
Oropharynx
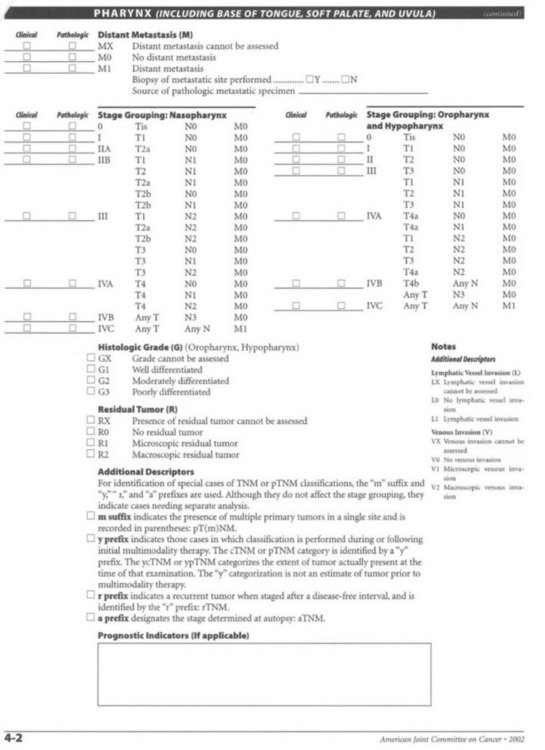
Staging (Table 17.2)
Table 17.2 TNM staging of the pharynx (including the base of the tongue, soft palate, and uvula)
Tonsil
The treatment of early tonsillar neoplasms (stages I and II) is usually radiation therapy as a single modality. Transoral wide local excision of small, superficial lesions may be locally effective, but does not address the high potential of occult lymph node metastasis, and thus a staging neck dissection should be performed if surgery is the primary treatment modality. While surgery and primary radiation offer comparable locoregional control for small tumors, many patients will require postoperative radiotherapy.47 A role for transoral robotic surgery is emerging for selected tumors of the oropharynx, particularly the tonsillar region. Surgical management of advanced cancers requires extensive resections of the pharyngeal wall or mandible,48,49 with free tissue transfer and postoperative radiotherapy. Patient function after intensive treatment is often poor, with a significant number dependent upon gastrostomy tube for nutrition and a tracheostomy for pulmonary toilet. Thus, a shift toward nonsurgical management with combined chemotherapy and radiotherapy approaches for tonsillar cancers has prevailed. However, radiation tends to have less short-term morbidity but significantly worse long-term morbidity, especially progressive radiation-induced fibrosis, which can cause wound-healing problems, long-term dysphagia, hoarseness, pain, or xerostomia that may also make future salvage operations and reconstructions more challenging. Stage III and IV tumors are most effectively treated with multimodality therapy, and some stage IV tumors are best treated with organ preservation protocols of chemotherapy and radiation. Surgery is often reserved for extensive disease and can result in severe deficits in speech and swallowing, as occur in the setting of a total glossectomy that may mandate a permanent feeding gastrostomy or tracheostomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree