and Ziv Gil2
(1)
Division of Otolaryngology Head and Neck Surgery and Maxillofacial Surgery, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
(2)
The Head and Neck Center Department of Otolaryngology Head and Neck Surgery, Rambam Healthcare Campus, Haifa, Israel
Keywords
Temporal boneMalignancyTemporal bone resection9.1 Introduction
Malignant tumors involving the temporal bone are rare, aggressive tumors, which carry substantial risks for morbidity and mortality. The annual incidence of primary cancer of the temporal bone is one to six cases per 1,000,000; males and females are equally inflicted. Common ages at presentation span the fifth and sixth decades. Diagnosis of patients with temporal bone cancers (TBCs) is often delayed, as early symptoms are often ascribed to the much more common otitis media or externa [1]. This delay is likely to have a detrimental effect on treatment and outcome.
The first description of a malignant temporal bone tumor appeared in Politzer’s 1883 A Text-book of the Diseases of the Ear and Adjacent Organs [2]. In the first half of the twentieth century, treatment choice included extended mastoidectomy followed by radiation therapy. During the second half of the twentieth century, en bloc subtotal temporal bone resection (STBR) and total temporal bone resection (TTBR) were described, and the techniques were refined [3–5]. In the past 30 years, the less morbid lateral temporal bone resection (LTBR) and its modifications became the mainstay of treatment for many lesions involving the temporal bone. Advancements in surgical and reconstructive techniques have allowed adequate tumor ablation and restoration of cosmesis and function [6]. Treatment of these uncommon and aggressive neoplasms is best managed by a multidisciplinary team comprising otolaryngologists, reconstruction surgeons, neurosurgeons, and oncologists. Ideally, the otologist is experienced in treating TBC [7].
Tumors can be primary, originating within the temporal bone, or they can invade the temporal bone from adjacent structures. It is useful to differentiate malignant tumors originating from the pinna from those arising in the temporal bone, although in advanced stages, both may converge to a similar-appearing lesion. If detected early in the course of the disease, a cancer occurring in the pinna is curable with adequate resection. The most common cancer of the pinna is basal cell carcinoma (BCC), followed by squamous cell carcinoma (SCC). The main risk factor for BCC and SCC of the pinna is sun-related actinic damage to the skin. When diagnosed and treated early, auricular SCC and BCC have an excellent prognosis.
In addition to SCC and BCC, malignant tumors of the external ear include adnexal carcinoma (ceruminous adenocarcinoma and adenoid cystic carcinoma [8]) as well as the exceedingly rare malignant melanoma, Merkel cell carcinoma, angiosarcoma, and lymphoma [9]. SCC is the most common tumor type in the external auditory canal (EAC). Risk factors for SCC of the EAC are exposure to ionizing radiation and chronic purulent otitis. Close to 20 % of malignancies of the EAC are of glandular origin. Malignant tumors of the middle and inner ear include metastatic carcinoma, endolymphatic sac tumors, primary squamous carcinoma, adenocarcinoma, rhabdomyosarcoma, lymphoma, multiple myeloma, and plasmacytoma [9]. The differential diagnosis includes chronic inflammatory and infectious diseases. Pseudoepitheliomatous hyperplasia is a very rare but benign epithelial reaction to inflammatory irritation that can be difficult to distinguish from SCC by physical and histopathological examinations [10]. Pseudoepitheliomatous hyperplasia does not require the aggressive treatment warranted by SCC. In the pediatric population, soft tissue sarcomas (mostly rhabdomyosarcomas) are the most common tumors. Other tumors that are relatively more common in this age group include eosinophilic granuloma and Langerhans cell histiocytosis.
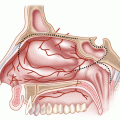
Cancer of the EAC can spread to neighboring structures [11]. Anteriorly, the tumor can extend through the cartilaginous canal into the parotid, through the concha to the postauricular sulcus, and through the tympanic membrane to the middle ear. Posteriorly, the tumor can extend from the middle ear, to the mastoid, to the carotid canal and eustachian tube. Tumors may enter the inner ear through the round window or otic capsule and extend along the facial nerve to the infratemporal fossa, jugular fossa, carotid artery, and lower cranial nerves.
9.2 Evaluation
A thorough history of the present illness and past medical and surgical histories is obtained. In addition to the relevant risk factors, the patient is asked about symptoms of cranial nerve dysfunction, headaches and earaches, trismus, and ear bleeding or discharge. Risk factors for microvascular disease are identified. The classic triad of signs of TBC comprises otorrhea, pain, and bleeding [12]. According to Gidley et al. [13], the four most common presenting complaints reported by patients with TBC, in descending order of frequency, are otorrhea (62 %), otalgia (52 %), hearing loss (44 %), and facial nerve dysfunction (15.5 %). Aural mass can be present in more than one-third of the patients [14]. Because these symptoms are also common in chronic infections, a critical delay in diagnosis is common [1].
Physical examination should include a meticulous survey of the auricular and periauricular skin. The neck and parotid gland are palpated to locate any nodal metastases. The function of the facial nerve and other cranial nerves is evaluated. Otoscopy is used to define the extension of the tumor in the EAC. If the drum can be seen, its integrity is verified. Jaw movement and trismus are examined as a potential sign of temporomandibular extension of the tumor. Sites of potential donor tissue to be used for reconstruction are examined. Physical examination is supplemented by imaging.
A high-resolution temporal bone CT scan with contrast is used to assess tumor extension. Particular attention is directed to these structures: EAC bone and cartilage, middle ear, tegmen of the attic and mastoid, bone plate bordering the posterior fossa, and temporomandibular joint (TMJ). The location of the sigmoid sinus and jugular bulb in relation to the annulus should be noted. The proximity of the auditory canal to the tegmen of the attic is important when LTBR is planned. Gadolinium-enhanced MRI is helpful in assessing soft tissue involvement and for evaluating the dura or the parotid gland. In one publication, MRI did not change the staging of patients, but provided additional information about soft tissue extension [15]. Regional lymph node status is assessed with ultrasound, CT scans, or MRI. PET-CT can be useful for identifying regional and remote metastases.
An audiogram is part of the pretreatment evaluation. The hearing status of the contralateral ear is established, and a hearing aid should be fitted to that ear as needed, especially if treatment is expected to compromise hearing in the diseased ear. Assessment of comorbidities can better prepare patients for the extensive surgical and adjuvant interventions and, in certain cases, may influence the treatment plan. In all suspected cases, tissue biopsy is a critical step in the diagnosis. Biopsy should include both the suspected lesion and neighboring healthy tissue.
9.3 Staging
Accurate staging of the disease is crucial for appropriate treatment planning and for sharing data. Multiple staging systems have been proposed and reported in the literature. Lately, it seems that the University of Pittsburgh updated TNM system is becoming the standard staging system (Table 9.1) [11, 13]; it will be used throughout this chapter. This system is likely to correlate well with prognosis for both squamous cell and non-squamous cell carcinoma [16]. Staging can be made on the basis of preoperative physical examination and imaging. An updated version up-staged patients with facial nerve paralysis from T3 to T4 [11], reflecting the detrimental effect of facial nerve dysfunction on prognosis [17, 18]. A good correlation between preoperative staging based on imaging and pathological staging has been confirmed [16]. Imaging may underestimate the extent of tumor spread, however [17], as tumors are known to spread along vascular and neural structures and through natural defects in bone and cartilage [19, 20]. High-quality MRI scans may help to resolve this issue. The presence of regional metastatic lymph nodes is an ominous sign for prognosis. Patients with regional metastases are assigned to stage III or IV.
Stagea | CT or pathological finding |
|---|---|
T1 | Tumor limited to the EAC without bony erosion or evidence of soft tissue extension |
T2 | Tumor with limited EAC bony erosion (not full thickness) or radiological finding consistent with limited (<0.5 cm) soft tissue involvement |
T3 | Tumors eroding the osseous EAC (full thickness) with limited (<0.5 cm) soft tissue involvement or tumor involving the middle ear and/or mastoid |
T4 | Tumor eroding the cochlea, petrous apex, medial wall of the middle ear, carotid canal, jugular foramen, or dura or with extensive (>0.5 cm) soft tissue involvement such as involvement of the TMJ or styloid process, or evidence of facial nerve paralysis |
N status | Involvement of lymph nodes is a poor prognostic finding and automatically places the patient in the advanced stage (i.e., stage III [T1N1] or stage IV [T2, T3, or T4 and N1]) |
M status | Distant metastasis indicates poor prognosis and immediately places a patient in the stage IV category |
9.4 Treatment
Once staging of the disease has been completed and extension of disease established, a treatment plan is outlined. Complete extirpation should be achieved aggressively whenever cure is the goal of treatment. The chance of curing a patient with recurrent or residual disease is lower than the chance of curing a patient with a primary lesion [13, 21, 22]. The main cause of death is local recurrence, rather than regional or metastatic disease [17, 21]. The treatment plan should address several questions:
Is the goal of treatment cure or palliation?
What is the appropriate extent and nature of surgical resection?
Is postoperative adjuvant therapy with radiation or chemoradiation anticipated?
Which reconstruction techniques will be employed?
9.4.1 Surgery
Nomenclature of surgery for TBC has lacked uniformity. This chapter uses the definitions presented in Table 9.2 [23]. Sleeve resection is a resection of part of the soft tissues of the EAC. LTBR is an en bloc resection of the EAC and its associated structures, including the malleus and tympanic membrane. In partial or selective LTBR, the medial aspect of the canal (usually the drum, engulfed by its annular ring) is spared. TTBR is the removal of the entire temporal bone; STBR is the same as TTBR with the exception of the carotid artery encased in its bony canal [3, 4, 6]..
Surgery | Specimen resected | Boundaries |
|---|---|---|
Sleeve resection | Skin of EAC | Tympanic membrane intact |
LTBR | En bloc removal of cartilaginous and bony EAC, tympanic membrane, malleus, and incus | Medial: stapes, middle ear cavity |
Posterior: mastoid cavity | ||
Superior: epitympanum, zygomatic root | ||
Comment: usually includes parotidectomy and selective neck dissection | Anterior: TMJ capsule | |
Inferior: medial tympanic bone, infratemporal fossa | ||
Optional: mandibular condyle | ||
STBR | Same as LTBR plus piecemeal or en bloc removal of tumor in the middle ear or mastoid, otic capsule, and medial aspect of middle ear | Petrous apex |
Neurovascular structures of the jugular foramen and carotid canal | ||
Dependent on spread: facial nerve, dura, brain tissue, contents of infratemporal fossa and sigmoid sinus | ||
TTBR | Same as STBR plus petrous apex | |
Dependent on spread: jugular foramen, carotid canal, dura, brain, other cranial nerves |
Sleeve resections are best reserved for small, superficial tumors of the canal skin that are easy to follow. These limited resections should be used judiciously. Partial LTBR can be used for extirpation of disease in select cases involving the lateral EAC, mostly when the tumor arises from the parotid gland. Leaving medially located skin (i.e., tympanic membrane) may pose a problem if a larger flap is used for reconstruction, as forming a sustainable lateral EAC is difficult. The trapped skin may become a source of ongoing inflammation.
Advanced-stage tumors require comprehensive surgical extirpation. An added benefit for TTBR or STBR of the temporal bone over piecemeal excision has not been proved [15, 21]. In their published results of piecemeal resection of TBC (tumor removed by fragments) followed by radiation therapy, Zhang et al. [24] have found it to be comparable to the previously published outcome of en bloc resections. En bloc STBR and TTBR require additional exposure, which may cause further morbidity. Consequently, it is our policy to use the piecemeal technique for advanced-stage tumors. The tissues surrounding the tumor are removed as well, in an effort to leave uninvolved, healthy tissue at the boundaries of extirpation. Resection almost always includes a composite removal with segmental mandibulectomy, parotidectomy (superficial or total), and neck dissection (if indicated). Other structures that may be resected are the infratemporal fossa, zygoma, pterygopalatine fossa, calvarium, and dura. Dura and brain involvement is not a contraindication for surgery with curative intent [21]. On the other hand, it is largely accepted that carotid artery involvement and significant brain involvement are contraindications for surgery. Metastases to regional lymph nodes (either in the neck or parotid gland) are found in 10–25 % of patients at presentation [25]. The most common nodes to harbor metastases in the neck are found at level II [11]. The status of neck nodes can guide decisions on adjuvant radiation therapy [25]. Neck dissection also facilitates control of carotid bleeding and free-flap reconstruction.
LTBR is the most extensive en bloc procedure that does not result in significant morbidity and risk of mortality. Disease-free survival with LTBR is comparable to the rate for more radical surgical therapy [18]. Hence, for most tumors originating from or protruding to the EAC, LTBR will be the procedure of choice.
9.4.1.1 Lateral Temporal Bone Resection
LTBR is the mainstay of treatment of malignant tumors involving structures of the EAC. It allows en bloc removal of the canal and adjacent structures. The extent of excision of the auricle, adjacent skin, and adjacent structures is adjusted according to the specific tumor and the patient’s characteristics. In addition to the obvious oncological advantages, histological examination of the specimen can reliably establish the true status of the margins of excision. If the integrity of the tympanic membrane is compromised during surgery, the status of the medial boundary of the specimen may remain undetermined, especially if the tumor involves the medial aspect of the EAC.
The patient is placed in a supine position suitable for temporal bone surgery. Facial nerve integrity monitoring is used when nerve preservation is a goal. The use of paralytic agents during anesthesia is adjusted accordingly. Perioperative antibiotics are advised.
1.




Planning skin incisions. Skin incisions and soft tissue approaches may vary according to the exact nature of the procedure. Most commonly, LTBR will be part of a more comprehensive surgery that may include parotidectomy and neck dissection. A retroauricular skin incision is used. This incision may be extended inferiorly beyond the mastoid tip. If the auricle or parts of it are to be preserved, considerations for the blood supply are part of the skin incision planning. Skin incisions should also accommodate reconstructive efforts, allowing access to local flaps, as required. For tumors involving the meatus, a circular area of auricle engulfing the meatus is incorporated with the specimen. The incision for this area is performed at the beginning of the procedure, as it is carried to the deep subcuticular tissues. The meatus is closed with sutures to minimize the risk of tumor spillage. If the auricle and neighboring skin require removal, they can be left attached to the EAC skin, the parotid gland, and the TMJ. From the retroauricular incision, a skin flap is elevated anteriorly toward the EAC; it can be merged with the circular incision made previously around the meatus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree