Breast Reconstruction with Free Tissue Transfer: An Algorithmic Approach
Julie E. Park
David H. Song
Background
Over the years, breast reconstruction has evolved from musculocutaneous flaps to muscle-sparing flaps to perforator flaps. These advances reflect the dual goals of minimizing donor-site morbidity and optimizing flap vascular perfusion. Several flaps have been developed that focus on essentially the same donor site—the lower abdominal fat and skin. These flaps can be harvested as a transverse ellipse, leaving a donor site similar to an abdominoplasty.
Carl Hartrampf (1) popularized the use of the lower abdominal skin and fat for breast reconstruction based on the superior epigastric vessels in a pedicled transverse rectus abdominus myocutaneous (TRAM) flap. The pedicled TRAM flap sacrifices a rectus muscle to perfuse the breast reconstruction. This can lead to abdominal wall weakness as well as abdominal bulges or hernias (2,3,4). This is especially problematic for bilateral TRAM flaps. Fascia-sparing techniques have been developed to preserve more fascia for reconstruction of the abdomen.
As microsurgery became more prevalent and reliable, the free TRAM procedure was developed. While still sacrificing a significant portion of the rectus muscle, this flap is based on the deep inferior epigastric vessels, which are the dominant blood supply to these tissues. This provided better perfusion to the flap and decreased partial flap necrosis, but increased the risk of complete flap failure due to microsurgical complications.
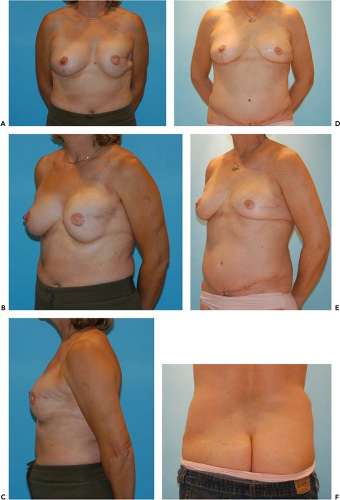
Advances in microsurgical techniques enabled a greater versatility in TRAM-based reconstructions. Perforator-based microsurgical techniques were applied to TRAM flaps, rooted in the angiosome concept of Taylor and Palmer (5). It became clear that muscle was a conduit for the blood supply but was not itself necessary for perfusion. Consequently, the free TRAM was trimmed to a muscle-sparing technique, and various gradations of muscle-sparing free TRAM (MS-TRAM) flaps are now used (6,7). The deep inferior epigastric perforator flap (DIEP) is isolated on the perforator alone with only a single myotomy made parallel to the rectus muscle fibers to minimize damage to the rectus muscle (8,9). The most-muscle-sparing version is based on the superficial inferior epigastric artery (SIEA) (10,11,12,13). This does not violate the fascia or muscle and is purely an adipocutaneous flap (Fig. 60.1).
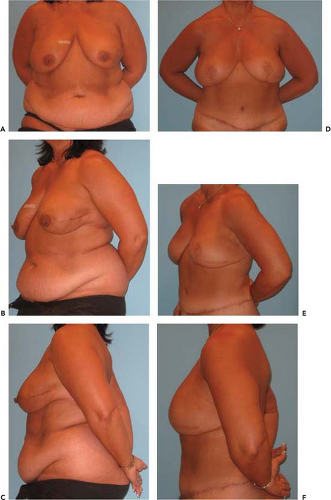
When there is a paucity of fat and skin available from the lower abdominal region, other secondary donor sites utilizing the same muscle-sparing principles, perforator exploration techniques and flap development can be considered. In particular, fat and skin from the gluteal region either based on superior gluteal artery perforators (SGAP) (Fig. 60.2) or inferior gluteal artery perforators (IGAP) can be utilized for breast reconstruction. More recently, free tissue transfers based on the obturator system, with sacrifice of part or all of the gracilis muscle, have been successfully utilized as the transverse upper gracilis (TUG) flap.
With this arsenal of potential flaps, the question becomes how to determine which is the most appropriate flap for full autologous tissue breast reconstruction of a particular patient. Each flap has its pros and cons in the balance between minimizing donor-site morbidity and optimizing flap perfusion. Additional implant use in conjunction with flaps has been described and often utilized but remains beyond the scope of our discussion of full autologous tissue reconstruction.
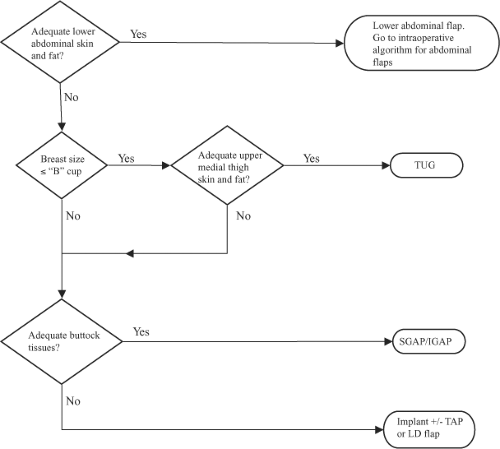
Algorithm
The donor site is chosen preoperatively (Fig. 60.3). First, the lower abdomen is appraised for adequate skin and subcutaneous fat. If this is available, then a lower abdominal flap (SIEA, DIEP, MS-TRAM) is chosen; the specific type of flap is decided using an intraoperative algorithm described later. If the lower abdomen is not available, then the medial thighs and buttocks are considered. If the breast reconstruction is relatively small, a B cup or less, and there are sufficient upper medial thigh tissues, then a TUG is chosen. If the tissues requirements exceed the thigh donor site, then the buttocks are evaluated. If there are adequate tissues, then a SGAP or IGAP is chosen. If there is insufficient buttock tissue, or if the patient does not desire the buttock donor site, then an implant reconstruction with or without thoracodorsal artery perforator (TAP) flap or latissimus dorsi (LD) myocutaneous flap is offered. The TAP and LD flaps are pedicled rotational flaps and usually require an expander/implant adjunct for volumetric balance and thus are beyond the scope of this chapter. Alternative flaps based on the circumflex iliac system (e.g., Ruben’s flap), the lateral femoral circumflex (anterolateral thigh), and omentum have also been described but are generally not used as first-line options.
The choice of flap for breast reconstruction using lower abdominal tissue is determined for the most part intraoperatively based on (a) the availability of recipient vessels, (b) tissue volume requirements, and (c) assessment of the donor-site vasculature (Fig. 60.4).
First the availability of recipient vessels needs to be determined. The internal mammary artery and vein are our preferred choice. When compared to thoracodorsal vessels, the internal mammary vessels allow for more medial insetting of the flap, typically are not sullied by the original extirpative operation, and are relatively less affected by scarring from adjunctive radiotherapy. Vessel diameters of 1.5 mm are sufficient. If the vessels are inadequate, then the thoracodorsal vessels are assessed secondarily. If this system is inadequate, then a pedicled TRAM
flap is chosen, or the case may be aborted and an alternative reconstruction may be planned for a later date. Heroic measures of arteriovenous loops using vein grafts with the carotid or thoracoacromial system can be utilized, but in our opinion, these should be discussed preoperatively and proper informed consent attained.
flap is chosen, or the case may be aborted and an alternative reconstruction may be planned for a later date. Heroic measures of arteriovenous loops using vein grafts with the carotid or thoracoacromial system can be utilized, but in our opinion, these should be discussed preoperatively and proper informed consent attained.
Once adequate recipient vessels have been established, next the tissue requirements for reconstruction of the breast envelope and volume need to be determined. A SIEA can support a hemiabdomen but does not reliably perfuse across the midline (14). If a SIEA has been determined to be an adequate flap for tissue needs, then the algorithm for determining the flap choice goes as follows.
First the superficial inferior epigastric system is examined. The SIEV should have a diameter of at least 1.5 mm. If the SIEV is not present or is too small, then the deep inferior epigastric system is interrogated. If an adequate vein is present, the superficial inferior epigastric artery is then assessed. More often than not, the artery is the vessel that is not present or is insufficient. It is present approximately 30% of the time and does not necessarily travel with the vein. The artery may be lateral to the vein up to 1 to 1.5 cm and typically lies just deep to the Scarpa’s fascia. While some centers have advocated size of 1.5 mm as a criterion for the SIEA, we have found that a palpable pulse is a reliable criterion for an acceptable artery. If both the artery and vein are adequate, then a SIEA flap is harvested as the flap of choice. If the vein is adequate but the artery is not, then the vein may be dissected as a long pedicle to use as a backup in case the flap would benefit from turbocharging.
If the SIEA is not sufficient, the flap is dissected in a suprafascial manner and the medial and lateral row perforators of the deep inferior epigastric system are identified. The artery needs to be visibly pulsating or palpable and the vein at least 1 to 1.5 mm in diameter. If a dominant perforator is located, then a DIEP flap is harvested (Fig. 60.5).
If there is not a single, dominant perforator, then the location and caliber of the perforators are assessed. If there are two or more medium-sized perforators in line with each other such that they fall within the same myotomy, a DIEP may still be harvested. If the perforators do not fall within the same myotomy, then a cuff of muscle may be harvested with the multiple perforators to perform a MS-TRAM. If there is no good collection of perforators, then a free TRAM may be performed.
Nahabedian et al. (15) described a numerical classification for MS-TRAM in which the number increases as the amount of muscle spared increases. Thus, a MS-0 TRAM harvests the full
width and partial length of the rectus abdominis muscle. The MS-1 TRAM harvests the medical portion of the rectus muscle but preserves the lateral segment (Fig. 60.6). The MS-2 TRAM harvests a central segment of muscle around the perforators but preserves both medial and lateral segments (Fig. 60.7). The MS-3 TRAM is essentially a DIEP, as it preserves the entire muscle. This classification was modified by Bajaj et al. (16) In this classification, the MS-1 TRAM is subdivided into MS-1-M, in which the medial portion of the muscle is preserved, and the MS-1-L, in which the lateral portion of the muscle is preserved.
width and partial length of the rectus abdominis muscle. The MS-1 TRAM harvests the medical portion of the rectus muscle but preserves the lateral segment (Fig. 60.6). The MS-2 TRAM harvests a central segment of muscle around the perforators but preserves both medial and lateral segments (Fig. 60.7). The MS-3 TRAM is essentially a DIEP, as it preserves the entire muscle. This classification was modified by Bajaj et al. (16) In this classification, the MS-1 TRAM is subdivided into MS-1-M, in which the medial portion of the muscle is preserved, and the MS-1-L, in which the lateral portion of the muscle is preserved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree