Breast Reconstruction: Prosthetic Techniques
Joseph J. Disa
Nima P. Patel
The use of prosthetic devices for breast reconstruction began in the early 1960s with silicone gel-filled implants. Over the years, implant technology and surgical techniques have evolved, resulting in improvement in the quality of the reconstructed breast. Currently, there are multiple methods of prosthetic breast reconstruction and various types of implants with different shapes, textures, and fill materials from which the plastic surgeon can choose.
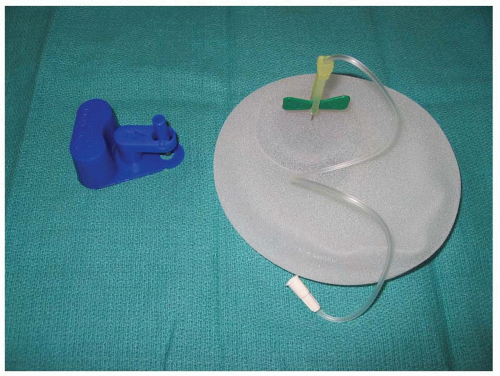
The popularity of one-stage implant reconstruction has diminished over the years with the development of two-stage, expander-implant reconstructions. Early experience with tissue expanders used smooth surface, round devices with remote fill ports. These devices were fraught with problems, including capsular contracture, poor expansion of the mastectomy pocket, and mechanical problems with the fill port. Current tissue expanders for breast reconstruction have textured surfaces, are anatomically shaped, and have integrated valves. These devices have a semirigid back, allowing for preferential expansion in the anterior dimension. Device design also provides preferential expansion in the lower pole of the reconstructed breast to create a better match with a natural breast. Finally, the textured surface on the expander reduces the incidence of capsular contracture (Figure 59.1). These expanders are typically made in varying heights, widths, degrees of projection, and shapes, so that the optimal device can be selected for the individual patient’s needs.1,2
PATIENT SELECTION
In general, most patients are candidates for prosthetic breast reconstruction. There are, however, limitations with the overall shape of permanent breast implants that dictate the quality of the final result. Factors to consider include unilateral vs. bilateral, body habitus, associated comorbidities, and the patient’s psychological profile. The ideal candidate for breast reconstruction with prosthetic implants is a thin patient with bilateral reconstruction, or a thin patient with a normal, nonptotic breast who requires unilateral reconstruction. In this situation, symmetry is relatively straightforward. As the patient’s breast size increases and the degree of ptosis increases, the difficulty matching the opposite side with prosthetic reconstruction increases. In this situation, the patient may be a candidate for a contralateral symmetry procedure such as a mastopexy and a reduction mammoplasty. Even with such procedures, however, exact symmetry out of clothing may not be possible. The patient is educated that the goal is to achieve as much symmetry as possible, but that this may only be accomplished when she is in her brassiere and clothing.3 Although not an absolute contraindication, obesity makes implant reconstruction difficult. In patients with a broad chest wall and a large contralateral breast, the expansion process may fail to achieve a pocket of appropriate volume to obtain a meaningful and symmetric result. In this situation, the addition of autologous tissue to an implant-based reconstruction, or the use of autologous tissue alone, may achieve a more pleasing result. For patients with multiple medical problems, an implant-based reconstruction may be more efficient than an autologous tissue reconstruction. However, implant-based reconstructions may require more than one operation and may require revisions over time. Additionally, if the patient has a chronic respiratory illness, the pressure from the tissue expander on the chest wall during the expansion process may exacerbate that underlying condition. Finally, prosthetic-based breast reconstruction often requires multiple steps and multiple visits to the office. The patients must be reliable and stable enough from a psychological standpoint that they can manage the reconstructive process.
It is also important to explain to patients that prosthetic breast reconstruction does not hinder detection of local or regional recurrence. There is no difference in the incidence of locoregional recurrence in patients who have undergone prosthetic reconstruction versus those who have not had reconstruction.4
TIMING
Breast reconstruction using prosthetic techniques can be accomplished either in the immediate or delayed setting. The advantage of immediate reconstruction is that the first step in breast reconstruction is accomplished at the time of the mastectomy under the same anesthetic. In this setting, maximum amounts of breast skin can be preserved as the prosthetic device will occupy some of the mastectomy space. In the setting of a single-stage breast reconstruction using a permanent implant, immediate reconstruction allows for the placement of an optimally sized device. Delayed breast reconstruction using a prosthetic technique is also possible; however, tissue expansion is almost always necessary. In this method, the mastectomy skin flaps are re-elevated and expanded to re-create a pocket for the ultimate placement of a permanent breast implant. In the setting of high-risk disease and patients who require chemotherapy and radiation therapy, a delayed reconstruction may be appropriate as it will not delay the initiation of adjuvant treatment.
TECHNIQUE
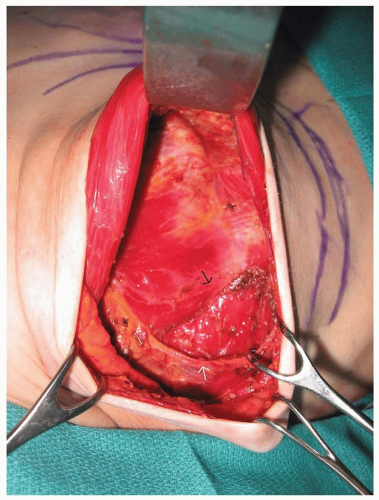
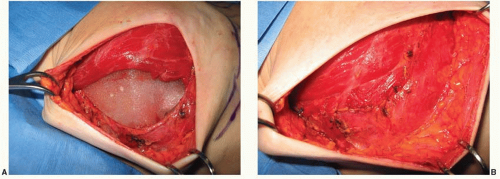
With all types of breast reconstruction, the primary goal is to achieve a breast mound that is as symmetrical as possible with the other breast or to the contralateral reconstruction in the setting of bilateral mastectomies. Coordination and communication between the surgeon performing the mastectomy and the reconstructive surgeon is required. Ideally, mastectomy incisions are planned to minimize their impact on subsequent tissue expansion and their visibility in conventional clothing. Skin flaps should be of adequate thickness to maintain blood supply, and the site of the inframammary fold should be marked and preserved whenever possible (Figure 59.2). The position of the point of maximum projection of the breast should also be noted. At the conclusion of the mastectomy, if the inframammary fold has been detached, it should be repaired. After obtaining hemostasis within the mastectomy pockets, a submuscular pocket for the placement of the tissue expander is prepared. The lateral border of the pectoralis major muscle is elevated from the chest wall and from the underlying pectoralis minor muscle. Care must be taken to adequately coagulate perforating vessels to the muscle to avoid hematoma formation. Expanders can be placed in a complete submuscular or subfascial pocket by elevating the medial border of the serratus anterior muscle and/or fascia and elevating the pectoralis major from lateral to medial and bringing both the subserratus and subpectoral pocket into communication at the level of or slightly below the inframammary fold (Figure 59.3). Final coverage of the expander occurs by suturing the lateral border of the pectoralis major muscle to the serratus anterior muscle. This technique completely separates the tissue expander from the mastectomy space (Figure 59.4A, B).5 In the setting of a very thin mastectomy skin flap, caution is recommended because there may be inadequate soft-tissue coverage over the inferior pole of the expander. Exposure of the expander either through the skin flap or through a poorly healed mastectomy incision does occur in some cases. In general, if soft-tissue coverage of the expander is questionable, any mastectomy skin flap necrosis should be treated aggressively with excision of devitalized tissue and closure of the wound. Occasionally, saline needs to be temporarily removed from the expander to accomplish closure.
An alternative to using the serratus anterior muscle and/or fascia for total submuscular coverage of the tissue expander is to use acellular dermal matrix. Once a subpectoral pocket is created for the expander, a sheet of acellular dermal matrix is tailored to the defect. It is placed in the inferior and lateral portions of the expander pocket and sutured to the pectoralis major muscle superiorly and to the chest wall or inframammary fold inferiorly (Figure 59.5). One to two closed suction drains are placed. Postoperative expansion starts in 10 to 14 days.
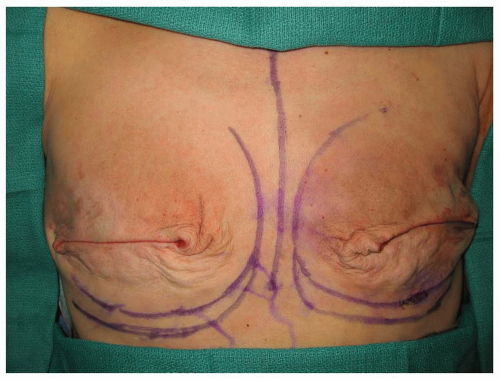 FIGURE 59.2. Intraoperative appearance of bilateral mastectomy defect. Original position of inframammary folds and planned lower position of the new inframammary fold are marked. |
It has been suggested in many case series that the use of acellular dermal matrix in tissue expander/implant reconstruction allows for quicker expansion, decreased pain caused by dissecting the serratus for submuscular coverage, and improved cosmesis. However, this is based on anecdotal reports. Initially, it was shown at our institution that there was no difference in the mean rate of postoperative tissue expansion with the use of acellular dermal matrix.6 More recently, however, increased rates of expansion due to larger initial fill volume in patients with acellular dermal matrix have been observed. Additionally, a recent study evaluated 153 immediate expanders placed using acellular dermal matrix versus 2,910 expanders placed without the acellular dermal matrix over a 4-year period. The acellular dermal matrix group had a higher incidence of overall complications, specifically seroma (7.2%) and reconstructive failure (5.9%) mostly due to infection.7
Choosing the appropriate expander is based on several factors, including breast volume, breast dimensions (height, width, and projection), breast shape, and the patient’s body habitus. In general, an anatomically designed, textured surface, integrated valve tissue expander is preferred (see Figure 59.1). The expander comes in various heights, widths, and amounts of projection that either can be compared with the contralateral breast or can be matched to another expander if a bilateral procedure is performed. Final considerations in choosing an
expander include the amount and quality of remaining breast skin after the mastectomy and the impact of planned contralateral symmetry procedures (augmentation, mastopexy, and reduction) on the shape of the opposite breast. The expander typically comes partially filled with air. The air is evacuated from the expander and a small amount of saline solution is infiltrated into the expander to confirm the functioning of the port. The expander is then placed into the pocket with the appropriate anatomic orientation. The expander can be filled to match the available space in the submuscular/submastectomy pocket. A closed suction drain is placed in the mastectomy space, and the mastectomy wounds are closed. If there is redundant skin from the mastectomy, excision of this skin as much as possible prior to closure will improve the cosmetic result.
expander include the amount and quality of remaining breast skin after the mastectomy and the impact of planned contralateral symmetry procedures (augmentation, mastopexy, and reduction) on the shape of the opposite breast. The expander typically comes partially filled with air. The air is evacuated from the expander and a small amount of saline solution is infiltrated into the expander to confirm the functioning of the port. The expander is then placed into the pocket with the appropriate anatomic orientation. The expander can be filled to match the available space in the submuscular/submastectomy pocket. A closed suction drain is placed in the mastectomy space, and the mastectomy wounds are closed. If there is redundant skin from the mastectomy, excision of this skin as much as possible prior to closure will improve the cosmetic result.
DELAYED TISSUE EXPANDERS
Delayed breast reconstruction with a tissue expander is similar to immediate reconstruction. Typically, the mastectomy scar is excised and the mastectomy flaps are re-elevated, although not to the extent as was necessary during the original mastectomy. Once adequate pectoralis muscle is exposed, either the lateral border of the pectoralis muscle is identified and elevated from the chest wall, or the muscle is split in the direction of the muscle fibers and a subpectoralis major pocket is created. Similar to immediate expander placement, care is taken to avoid elevation of the pectoralis minor muscle. From this point, dissection beyond the pectoralis can be extended either into the subcutaneous plane inferiorly and laterally, or into the submuscular/subfascial plane as noted in the description of placement of an immediate tissue expander. Similar to immediate tissue expander placement, the importance of a careful dissection of the tissue expander pocket cannot be overemphasized. It is critical to free any scar tissue that will restrict expansion of the mastectomy flaps. The expander is placed such that the zone of maximum expansion is located in the lower pole of the reconstructed breast, allowing for preferential expansion of the lower pole, for a more natural shape of the reconstructed breast. Acellular dermal matrix may also be used for delayed reconstruction.
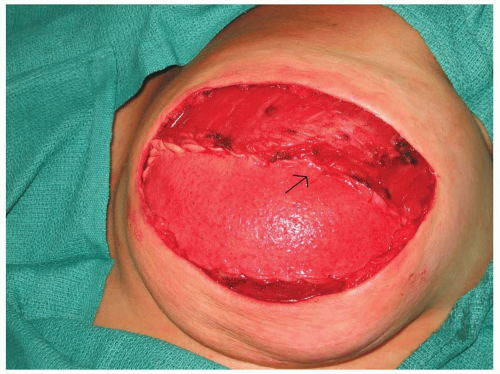 FIGURE 59.5. Tissue expander covered by the pectoralis major muscle superiorly sutured to the acellular dermal matrix (black arrow) inferiorly. |
Expansion
Intraoperatively, the tissue expander is filled to a volume that optimally obliterates dead space, but does not impart excessive pressure on the mastectomy skin flaps (Figure 59.6A-C). Because blood supply to the newly created mastectomy skin flap may be tenuous, overfilling the expander intraoperatively can impede circulation. Closed suction drainage tubes left at the time of expander placement are removed when output is ≤30 mL per 24 hours, which typically occurs within 2 weeks. Tissue expansion begins in the office approximately 10 to 14 days after surgery. A magnetic device is used to identify the site of the integrated fill valve under the patient’s skin. The area is cleansed with an antiseptic solution and a butterfly needle is used to gain access to the tissue expander. Approximately 30 to 120 mL of saline is injected into the expander during each expansion session. Expansion sessions can occur as frequently as once per week or as infrequently as once per month, although there is no set criterion to the expansion schedule. The final goal of the expansion is to achieve a volume that is approximately 25% to 30% greater than the expander volume (Figure 59.7). This allows for extra skin to be available at the exchange procedure, which can be used to create maximum breast ptosis and inferior pole projection. Overexpansion also allows for the removal of unsightly mastectomy scars, or scars that have resulted from delayed or poor wound healing. If the patient is to receive postoperative chemotherapy, the onset of this typically coincides with the expansion process. Patients can be safely expanded during chemotherapy, although it may be necessary to coordinate
the expansion schedule with their chemotherapy schedule. Final replacement of the expander to a permanent implant is deferred until the patient’s blood counts have returned to normal after the conclusion of chemotherapy. Also after simulation for radiotherapy, it is important not to adjust the expander volume. In general, soft tissues are allowed to rest for at least 1 month between the time of the last expansion and the time of the exchange procedure.8
the expansion schedule with their chemotherapy schedule. Final replacement of the expander to a permanent implant is deferred until the patient’s blood counts have returned to normal after the conclusion of chemotherapy. Also after simulation for radiotherapy, it is important not to adjust the expander volume. In general, soft tissues are allowed to rest for at least 1 month between the time of the last expansion and the time of the exchange procedure.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree