I. HISTORY
| 1896 | Tansini | Latissimus dorsi flap for post-mastectomy coverage |
| 1964 | Cronin and Gerow | Introduction of silicone implant |
| 1982 | Hartrampf | TRAM flap |
| 1983 | Taylor | DIEP flap |
| 1989 | Grotting | Routine use of free TRAM for breast reconstruction |
II. GOALS
A. Oncologic treatment comes first, breast reconstruction comes second
B. Meet the patients needs: Breast reconstruction is elective, patient preferences should play a large role in decision-making
III. PATIENT HISTORY: WHAT TO ASK AT THE FIRST CLINIC VISIT
A. Complete cancer history
1. Diagnosis (ductal carcinoma in situ, lobular carcinoma in situ, invasive ductal, etc.) and cancer stage
2. Size and location (left/right, quadrant)
3. Method of diagnosis (fine needle aspiration, biopsy, and radiographic)
4. Previous treatment with dates (lumpectomy, mastectomy, chemo, and radiation)
5. Planned treatment (sentinel lymph node biopsy, lumpectomy/mastectomy, chemo, and radiation)
B. Breast history
1. Previous breast diagnoses (cysts, masses, and cancer)
2. Previous breast surgeries (biopsies, reduction/augmentation, and lumpectomies)
3. Current breast size, desired breast size
C. Family history of breast cancer
1. First-degree relative
2. BRCA status
D. Past medical history, medications, past surgical history, and social history
1. Important to note medical conditions that could affect patients ability to withstand long operation or affect wound healing (coronary artery disease, diabetes, autoimmune disease, and bleeding diatheses)
2. Medications that affect bleeding/wound healing (coumadin, steroids, etc.)
3. Surgeries that affect the use of certain donor sites: Scars on abdomen, back, buttock, and thighs
4. Smoking status
IV. PHYSICAL EXAM
A. Height, weight, and BMI
B. Current breast size, symmetry of size/shape/inframammary fold (IMF) position, and nipple position
C. *Degree of breast ptosis
1. Grade 1: Nipple at IMF
2. Grade 2: Nipple below IMF
3. Grade 3: Nipple points straight down
______________
*Denotes common in-service examination topics
E. Palpable masses
F. Nipple retraction, ulceration, and discharge
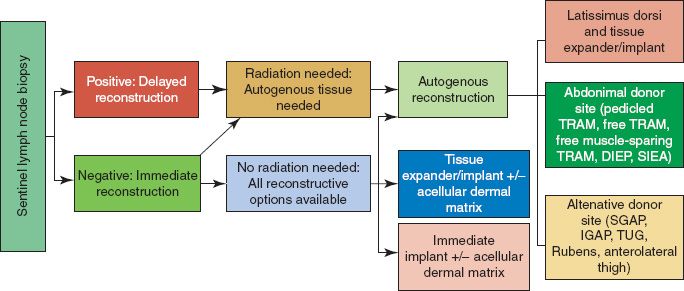
V. DECISION-MAKING ALGORITHM (FIG. 37-1)
A. Timing of reconstruction
1. Immediate: Reconstruction done at the same time as mastectomy
a. Early-stage disease
b. Low risk of needing radiation (based on cancer stage or negative sentinel lymph node biopsy)
2. Delayed: Reconstruction done after cancer treatment complete
a. Advanced stage disease
b. Known need for radiation
c. Patient preference
B. Type of reconstruction
1. Implant-based
a. Immediate implant: Placement of silicone or saline implant at the time of mastectomy
i. Few patients are candidates for this: Must have a large, good-quality skin envelope
ii. Patient must be willing to accept smaller breasts
iii. Often requires the use of large acellular dermal matrix for coverage of inferior implant
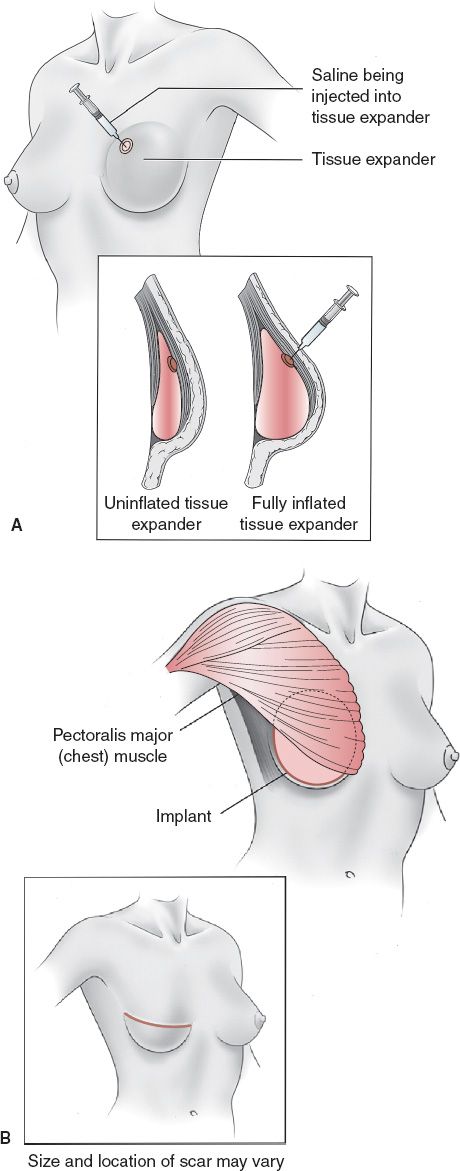
b. Tissue expander/implant: Placement of tissue expander first followed by implant exchange later (Fig. 37-2)
i. Most common type of reconstruction
ii. Tissue expander placed subpectorally. Inferolateral aspect of expander commonly covered by serratus fascia or acellular dermal matrix (trade names AlloDerm, Flex-HD, and AlloMax).
iii. Intraoperatively, may start filling TE if mastectomy flaps will tolerate without too much tension.
iv. If drains are placed, can start expansion 1 week after drain removal, usually 2 to 3 weeks post-op.
v. Expansions occur weekly, typically 60 to 120 cc saline injected each time, depending on the patient tolerance of expansion.
vi. Most patients require four to eight expansions depending on the size of expander and patients desired final size.
vii. Must overexpand by 10% or more past patients goal size to account for recoil at the time of expander removal.
viii. Once expansion is complete, one must wait for several months to allow the expanded skin to settle into new position prior to placing permanent implant.
Figure 37-1. General breast reconstruction algorithm.
Figure 37-2. Tissue expander-implant–based breast reconstruction. A: Process of tissue expansion. B: Placement of permanent implant after tissue expansion has completed. Note: Can use serratus muscle or acellular dermal matrix to cover the inferolateral aspect of the tissue expander/implant (not shown). (From Mulholland MW, ed. Greenfield’s Surgery. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.)
ix. At a second stage, tissue expander is exchanged for permanent silicone or saline implant
a) Revisions to pocket made at this time: Capsulotomies, IMF adjustments (resuspension or lowering as needed), and skin tailoring
b) For unilateral reconstruction, opposite breast surgery can be done at this time: Mastopexy, reduction, and augmentation
c. Complications
i. Early: Hematoma (0% to 5%), seroma (0% to 5%), infection (0% to 15%), mastectomy flap necrosis (0% to 21%), and expander/implant failure or extrusion (0% to 20%)
ii. Late: Implant rupture, capsular contracture, and visible wrinkling
iii. *Anaplastic large cell lymphoma (ALCL)
a) Very rare lymphoma may be associated with implants, <40 cases reported worldwide. Course is more benign than patients without breast implants who develop ALCL.
b) Patients who develop late seroma (>6 months from implant placement) should be worked up with aspiration and pathologic diagnosis.
2. Autologous
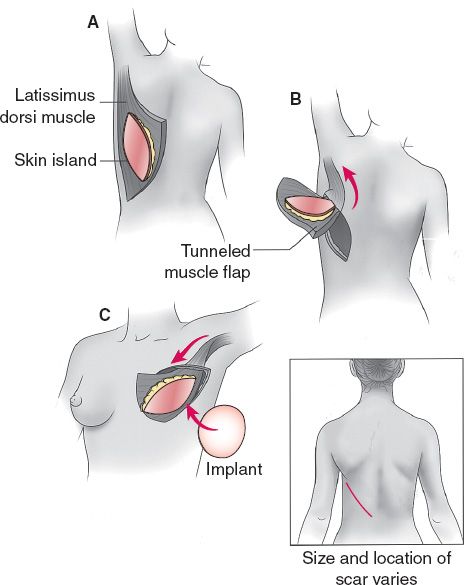
a. Latissimus dorsi ± tissue expander/implant (Fig. 37-3)
i. *Type V flap: Blood supply from (1) thoracodorsal artery (off subscapular) and (2) intercostal perforators. Thoracodorsal is primary blood supply for use in breast reconstruction.
a) Thoracodorsal artery enters latissimus muscle 8.7 cm distal to origin of subscapular artery and 2.6 cm medial to the lateral border of muscle
Figure 37-3. Latissimus dorsi flap reconstruction. A: A skin island overlying the latissimus dorsi muscle is designed, and the flap is elevated. B: The flap is tunneled through the axilla to the chest. C: The flap is inset on the chest, and an implant or tissue expander is placed. (From Mulholland MW, ed. Greenfield’s Surgery. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.)
b) Upon entry into latissimus, artery splits into lateral and medial branches.
c) If thoracodorsal vessels are damaged, latissimus can also survive on retrograde flow from serratus branch.
ii. Latissimus muscle is harvested with an elliptical skin paddle from the back (up to 10 cm wide will close primarily).
iii. “Workhorse flap”: Very reliable, easy to harvest, relatively short operative time, excellent option for patients who need autologous tissue but are not good candidates for larger operations.
iv. Volume of flap is small, almost always requires tissue expander/implant under flap to provide adequate breast size.
v. Complications: *Seroma (up to 50%), hematoma, capsular contracture, and partial flap loss.
b. Abdominal-based
i. TRAM: Transverse rectus abdominus myocutaneous flap
a) Rectus muscle plus transverse island of the skin from lower abdomen
b) *Type III flap: Dual blood supply from superior epigastric artery (off internal mammary artery [IMA]) and deep inferior epigastric artery (off external iliac).
c) Methods of harvest
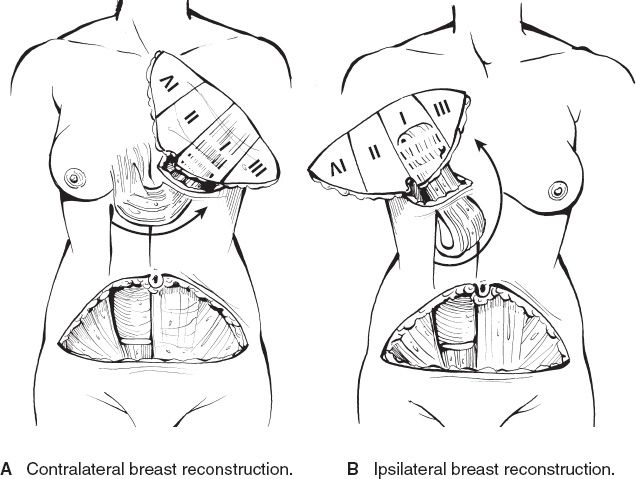
1) Pedicled TRAM (Fig. 37-4)
i) Based off superior epigastric artery
ii) Rectus muscle kept attached superiorly, deep inferior epigastric artery and inferior rectus attachments are disconnected.
iii) Muscle and skin flap are turned and passed through a subcutaneous tunnel at the IMF up to mastectomy defect to create new breast mound.
iv) Delay 1 to 2 weeks prior to operation by ligating deep inferior epigastric artery and vein might improve outcomes by increasing size and flow through superior epigastric vessels.
Figure 37-4. Pedicled TRAM flap. Zones I through IV are indicated and denote different areas of blood supply within the flap. (Modified from Vasconez LO, Lejeur M, Gamboa-Bobadilla M. Atlas of Breast Reconstruction. Philadelphia, PA: JB Lippincott; 1991:4.10, with permission.)
i) Rectus muscle is detached completely with transverse skin island and transferred to the chest as a free flap.
ii) Anastomosis between deep inferior epigastric and IMA or thoracodorsal.
iii) Advantage: Allows for greater manipulation of flap position on chest wall. Disadvantage: Longer operation, higher likelihood of flap complications versus pedicled.
3) Free muscle-sparing TRAM
i) Transverse abdominal skin is transferred with a strip of rectus muscle overlying blood supply, leaving behind most of rectus.
ii) MS-1: Preserves lateral segment of rectus; MS-2: Preserves medial and lateral segments of rectus.
ii. DIEP: Deep inferior epigastric perforator flap (see also Chapter 5)
a) Free transverse island of abdominal skin and subcutaneous tissue with no muscle.
b) Blood supply via perforators from deep inferior epigastric artery.
c) If lateral and medial perforators present, can place clamp on one or the other and observe which one leads to better looking flap or use intraoperative imaging of perfusion.
d) Microvascular anastomosis between deep inferior epigastric and IMA or thoracodorsal.
e) Advantage: Preserves entire rectus muscle. Disadvantage: Tedious dissection, higher likelihood of flap complications.
iii. SIEA: Superficial inferior epigastric artery flap
a) Free transverse island of abdominal skin and fat only with no violation of fascia.
b) Blood supply via SIEA off femoral artery.
c) <30% of patients have SIEA large enough to use this flap
d) Advantage: Complete preservation of rectus muscle and fascia; Disadvantage: Few patients are candidates, higher risk of fat necrosis/flap complications.
e) Complications: Partial/total flap loss, fat necrosis, delayed wound healing, abdominal hernia/bulge, wound infection, dehiscence, hematoma, and lymphedema.
f) Good to preserve these vessels even if not using it as the primary pedicle. SIEV can serve as a lifeboat if free TRAM or DIEP has venous congestion.
c. SGAP: Superior gluteal artery perforator flap (Fig. 37-5)
i. Free elliptical flap from upper buttock includes skin, subcutaneous tissue, but preserves gluteus maximus muscle.
ii. *Blood supply via perforators from superior gluteal artery (SGA) (off internal iliac).
iii. SGA emerges one-third of the way from posterior superior iliac spine to greater trochanter.
iv. Skin paddle up to 13 cm wide can be closed primarily
v. Advantages: Hidden donor site, good for thin patients with paucity of abdominal fat. Disadvantages: Buttock fat less malleable/harder to contour, difficult dissection requires experience.
vi. Complications: Contour deformity of the buttock, donor site seroma, fat necrosis, and partial/total flap loss
d. IGAP: Inferior gluteal artery perforator flap
i. Free flap from lower buttock includes skin, subcutaneous tissue, and small segment of gluteus maximus muscle overlying perforators.
ii. Blood supply via descending branch of inferior gluteal artery (off internal iliac).
iii. Much less commonly used versus SGAP
Figure 37-5. Superior gluteal artery perforator flap (SGAP).
e. TUG: Transverse upper gracilis flap
i. Free semilunar flap from upper thigh located one fingerbreadth below groin crease, centered over upper portion of gracilis muscle.
ii. Type II flap (gracilis): Blood supply via medial circumflex femoral artery (off profunda femoris) and venae comitantes, secondary supply to gracilis via superficial femoral artery perforators.
iii. Advantages: Hidden donor site, alternative for patients without adequate abdominal tissue. Disadvantages: Small flap volume, donor site complications.
iv. Complications: Donor site scarring, medial thigh sensory changes, fat necrosis, partial/total flap loss, and small reconstructed breast size.
f. Rubens/DCIA: Deep circumflex iliac artery flap
i. Free elliptical flap from soft tissue just above iliac crest.
ii. Blood supply via cutaneous perforators from deep circumflex iliac artery.
iii. Rarely used due to small size unless other options are unavailable.
g. ALT
i. Free elliptical flap from lateral thigh soft tissue.
ii. Blood supply via perforators from lateral circumflex femoral artery.
iii. Rarely used due to small size and conspicuous donor site.
VI. NIPPLE–AREOLAR RECONSTRUCTION
A. Commonly 2 to 3 months after final breast mound reconstruction complete
B. Papule reconstruction options
1. Nipple sharing: Graft from opposite nipple
2. Composite graft (ear and hallux)
3. Prosthetic material (AlloDerm and polyurethane)
4. Local flap (most popular): Uses local breast skin/fat to build papule. Many variations described, choice of technique is surgeon-dependent. Examples include
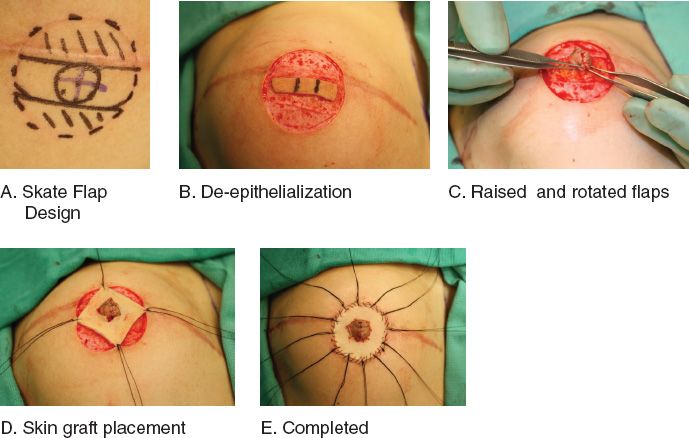
a. Skate flap (Fig. 37-6)
b. Other options: Star flap, Bell flap, S-flap, H-flap, double-opposing tab flap
5. Biggest problem with all techniques is loss of papule projection, 50% or more, after 1 year. Many surgeons will overcompensate initially to account for loss over time.
Figure 37-6. Nipple–areolar reconstruction with Skate flap (A-E).
C. Areola reconstruction
1. Tattoo: Pigment applied to simulate areola
2. Skin graft: Doughnut-shaped full-thickness skin graft applied around papule gives color and texture contrast
3. Can also do in situ skin graft by lifting the skin surrounding papule and then sewing back down
PEARLS
1. The choice of reconstructive option depends on multiple factors: Surgeon experience/preference, patient preference, and patient specifics (BMI, age, and history of XRT)
2. Two initial pieces of information can quickly narrow available options—History/plan for XRT (must use autologous tissue, or TE/implant with latissimus “protection”; and amount of infraumbilical lipodystrophy (too little or too great can negate use of TRAM variations)
3. Reconstruction always comes second to adequate cancer treatment
4. Check the continuity of the thoracodorsal artery when raising a latissimus flap in a patient who has had an axillary dissection. The muscle can possibly still be pedicled on the serratus branch (know arterial tree in this location) if necessary.
5. Do not hesitate to delay immediate reconstruction if there is questionable viability of the mastectomy flaps: Flap necrosis over a reconstruction can be devastating
QUESTIONS YOU WILL BE ASKED
1. What is the blood supply to the TRAM flap?
Type III classification: The superior (pedicled) or inferior (free or DIEP variations) epigastric arteriovenous pedicles.
2. Latissimus?
Type V classification: The thoracodorsal pedicle, and/or lumbar perforators.
3. Name some options for nipple reconstruction, and be able to draw them.
Skate flap, star flap, H-flap, etc.
THINGS TO DRAW
Markings for a nipple reconstruction flap
See Figure 37-6
Recommended Readings
Dancey A, Blondeel PN. Technical tips for safe perforator vessel dissection applicable to all perforator flaps. Clin Plast Surg. 2010;37(4):593–606, xi-vi. PMID: 20816515.
Shestak KC, Gabriel A, Landecker A, Peters S, Shestak A, Kim J. Assessment of long-term nipple projection: a comparison of three techniques. Plast Reconstr Surg. 2002;110(3):780–786. PMID: 12172139.
Sigurdson L, Lalonde DH. MOC-PSSM CME article: breast reconstruction. Plast Reconstr Surg. 2008;121(1 Suppl):1–12. PMID: 18182962.
< div class='tao-gold-member'>