ANATOMY AND DEVELOPMENT OF THE BREAST
I. GLAND
A. Boundaries
1. Superior: Second rib or clavicle
2. Inferior: Sixth rib
3. Medial: Sternal edge
4. Lateral: Midaxillary line, extends into axilla as axillary tail of Spence
5. Posterior: Fascia of pectoralis major superiorly and medially, fascia of serratus anterior inferiorly and laterally
B. Composition: Skin, fat, and glandular tissue
1. 10% to 15% epithelial; remainder is stromal
a. Large proportion of epithelial tissue found in upper outer quadrant, the most common site for both benign and malignant disease.
b. 15 to 20 radially arranged glandular lobes, supported by fibrous connective tissue with varying amounts of adipose tissue in between the lobes.
c. Lobes are subdivided into lobules, then into tubuloalveolar glands.
d. Each lobe concludes as a lactiferous duct, which are 2 to 4 mm in diameter
e. Lactiferous ducts dilate into lactiferous sinuses beneath the nipple, then open through small orifices onto the nipple.
C. Nipple–areolar complex (NAC)
1. Located at the fourth intercostal space in nonptotic breasts
2. Composed of sebaceous glands and apocrine sweat glands
3. Montgomery glands: At the areolar periphery, capable of secreting milk
4. Tubercles of Morgagni: Elevations formed by the openings of Montgomery glands
5. Radial smooth muscle fibers beneath the nipple contribute to nipple erection
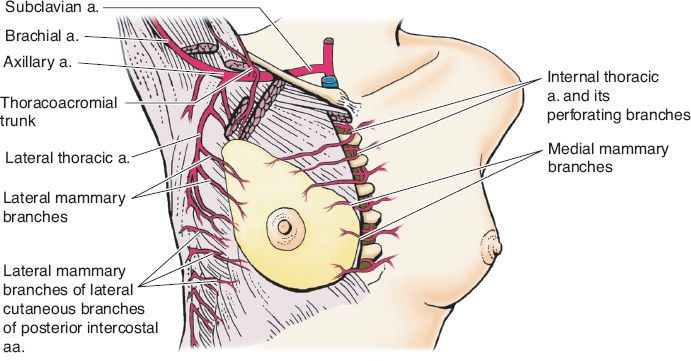
II. BLOOD SUPPLY (Fig. 35-1)
A. *Internal mammary artery: Perforating branches supply the medial and central portions of the breast; dominant blood supply of the breast and NAC.
B. Lateral thoracic artery: Upper outer quadrant
C. Anterolateral and anteromedial intercostal perforators
D. Venous drainage: Follows arterial supply
III. INNERVATION
A. Medial: Second through fifth anteromedial intercostal nerves.
B. Lateral: Third through sixth anterolateral intercostal nerves.
C. *NAC: Lateral cutaneous branch of the fourth intercostal nerve.
IV. LYMPHATIC DRAINAGE
A. Skin, nipple, and areola: Superficial subareolar lymphatic plexus
B. Breast: Deep lymphatic plexus, which is connected to the superficial plexus. About 97% of the breast drains directly into the axillary nodes, while the rest drains into the internal mammary nodes.
______________
*Denotes common in-service examination topics
Figure 35-1. Blood supply to the breast (From Moore KL, Dalley AF, Agur AM, eds. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
C. Axillary space
1. Borders
a. Axillary vein
b. Serratus anterior
c. Latissimus dorsi
d. Pectoralis major
e. Subscapularis
2. Structures of clinical importance within the axilla
a. Long thoracic nerve: Innervates serratus anterior
b. Thoracodorsal neurovascular bundle: Innervates and supplies latissimus dorsi
c. Intercostobrachial nerve (sensation to upper medial arm)
3. Axillary lymph nodes: Classified by relationship to pectoralis minor
a. Inferior and lateral to pectoralis minor. Deep to pectoralis minor and inferior to axillary vein. Medial to pectoralis minor and against the chest wall
b. Interpectoral nodes, between the pectoralis major and minor, along the lateral pectoral nerve.
D. Supraclavicular nodes: Contiguous with axillary apex
E. Internal mammary nodes: In first six intercostal spaces with highest concentration of nodes in first three spaces, within 3 cm of sternal edge
V. DEVELOPMENT
A. Tanner stages of breast development
1. Stage I: No glandular tissue; prepubertal
2. Stage II: Breast bud begins to form, areola begins to widen.
3. Stage III: Breast becomes more elevated, extends beyond borders of areola, which continues to widen but remains in contour with surrounding breast.
4. Stage IV: Increased breast size and elevation, areola and papilla form a secondary mound projecting from the chest contour.
5. Stage V: Breast reaches the final adult size, areola returns to contour of surrounding breast with a projecting central papilla.
B. Menopause: Involution of ductal and glandular elements, breast becomes predominantly fat and stroma.
CLINICAL EVALUATION OF BREAST MASSES
I. HISTORY
A. Onset and duration of lesion
B. Pain, change in breast size or shape, nipple discharge, skin changes, weight loss, and/or fatigue
C. Family history of breast cancer, including relationship, age of onset, and presence of bilateral disease
D. Change in size with menstrual cycle or estrogen exposure
E. Estrogen exposure: Timing of menarche, pregnancy and menopause, and history of oral contraceptives or hormone replacement therapy (HRT).
F. History of previous breast biopsy or other breast surgery
II. PHYSICAL EXAMINATION
A. Patient in supine, raise arm above the head to examine the breast
B. Patient seated upright, support arm to relax pectoralis and examine axilla
C. Masses and nodes are characterized by their location, number, size, firmness, and mobility
BENIGN BREAST DISEASE
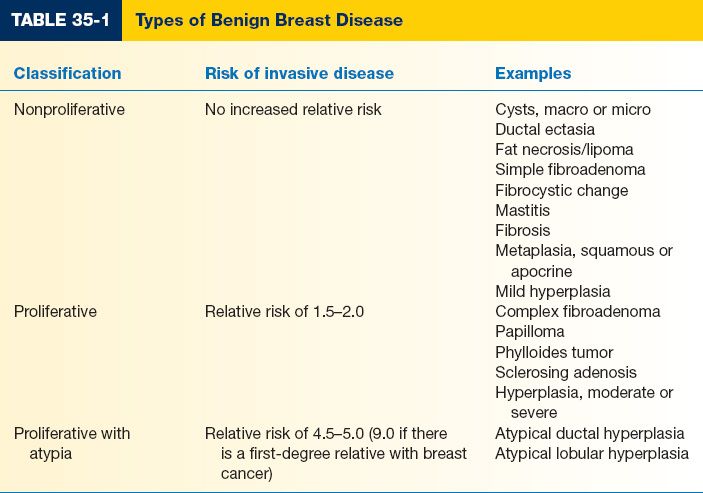
I. BENIGN BREAST DISEASE TYPES (TABLE 35-1)
A. Nonproliferative
1. Most common: 70% of palpable breast masses
2. No increased risk of breast cancer
B. Proliferative. Small increase in relative risk (1.5 to 2.0) for developing invasive breast cancer
C. Proliferative with atypia
1. Less common: 3.6% of palpable breast masses, 7% to 10% of nonpalpable breast abnormalities
2. Relative risk of 4.0 to 5.0 for developing invasive cancer
3. Relative risk increases to 9.0 with a first-degree relative with breast cancer
II. NONPROLIFERATIVE BENIGN BREAST DISEASE
A. Cysts
1. Epidemiology: Perimenopausal, ages 40 to 50
2. Etiology: Lobular involution; acini within the lobule distend to form microcysts, which develop into macrocysts
3. Presentation
a. Well demarcated from the surrounding tissue, mobile, and firm
b. May fluctuate with patient’s menstrual cycle (solid lesions do not)
c. Uncommon in postmenopausal women who are not on HRT
4. Diagnosis/management
a. Ultrasound differentiates simple cysts versus complex cysts
b. Simple cyst: If asymptomatic, may be followed. If symptomatic, should be aspirated. If nonpalpable, no aspiration needed.
c. Complex cyst: Biopsy
d. Aspiration: May replace ultrasound as the initial step
i. If aspirate is nonbloody and mass resolves, no further treatment needed. If fluid is bloody, mass does not resolve completely, or if it recurs multiple times, should be biopsied.
ii. Cytologic analysis of fluid is of little value as malignant cells are seen in <1% of cases and atypical cells are often present even in benign disease
e. Surgical biopsy
i. Indications: Recurrent, bloody, persistent, or complex cysts
ii. Cyst wall tissue is required to determine the presence of malignancy
B. Ductal ectasia
1. Epidemiology: Perimenopausal, ages 40 to 50
2. Etiology: Chronic intraductal and periductal inflammation causes dilation of mammary ducts and thickening of secretions, leading to a blockage of the ducts
3. Presentation
a. Palpable subareolar mass
b. Nipple tenderness, irritation, or retraction
c. Thick black, gray, or greenish nipple discharge
4. Diagnosis/management
a. Imaging: Ultrasound or mammography
b. Biopsy for definitive diagnosis
c. Once diagnosed, no further treatment is needed as condition usually resolves on its own. However, if symptoms recur, excision of the affected duct may be indicated.
d. If duct becomes infected, treat with antibiotics
C. Fat necrosis
1. Epidemiology: Most common in women with pendulous breasts or who are overweight
2. Etiology: Secondary to trauma, breast surgery, infection, or radiation therapy; 50% are idiopathic
3. Presentation
a. Poorly defined, painless, firm mass
b. Usually occurs in superficial breast tissue
c. May be accompanied by skin thickening, dimpling, or retraction
4. Diagnosis/management
a. Mammography for diagnosis; however, diagnosis can only be made radiographically if an oil cyst (circumscribed mass of mixed soft-tissue density and fat with a calcified rim) is seen
b. Biopsy required to exclude malignancy in the absence of this finding or a clear history of trauma
D. Fibroadenoma
1. Epidemiology
a. Ages 15 to 35
b. *Account for 75% of breast biopsies in women younger than age 20.
a. Aberrant lobular development; proliferation of epithelial and fibrous tissues
b. Hormonal factors play a role in growth—fibroadenomas significantly increase in size during pregnancy and involute after menopause
3. Presentation
a. Well-defined, palpable mass that averages 2 to 3 cm diameter
b. Rubbery texture, mobile
c. Painless, slow-growing
d. Solitary in 85% to 90% of cases
4. Diagnosis/management
a. Ultrasound: Round or oval, well-circumscribed, solid, homogenous mass
b. Biopsy, excision. Pathology typically will be “simple” or “complex”—influences the risk of breast cancer
5. Types
a. Simple fibroadenoma: Classified as nonproliferative breast disease, no proliferative histologic features.
b. Complex fibroadenoma
i. Classified as proliferative breast disease and is associated with an increased risk of breast cancer.
ii. Contains cysts >3 mm in diameter and/or other proliferative changes, such as sclerosing adenosis, epithelial calcifications, and papillary apocrine changes.
c. *Giant fibroadenoma
i. Most common breast neoplasm in an adolescent patient
ii. Solitary, firm, and nontender
iii. Presents near puberty as rapid, asymmetric breast enlargement with prominent veins over tumor and occasional skin ulceration due to pressure
iv. Size >5.0 cm
v. Excise by enucleation, no adjuvant treatment indicated
E. Fibrocystic changes or disease
1. Epidemiology: Most common benign breast condition, affecting women during reproductive years or if taking hormone replacements after menopause.
2. Etiology: Related to fluctuations in hormone levels
3. Presentation: Cyclic, bilateral breast pain and tenderness associated with nodularity that occurs most commonly in upper outer breast quadrant, symptoms peak just prior to menstruation.
4. Diagnosis/management
a. Observation for symmetrical tender nodularity
b. If asymmetric nodularity persists for one to two menstrual cycles, order mammogram and biopsy for definitive diagnosis.
c. Lifestyle changes are beneficial to some patients, including restricting caffeine and methylxanthines and adhering to a low-salt diet.
d. Bilateral mastectomy may be indicated in patients with intractable pain.
F. Mastitis in lactating women
1. Epidemiology: Most common during the first 4 to 6 weeks postpartum or during weaning.
2. Etiology: Proliferation of bacteria, most commonly Staphylococcus aureus, in poorly drained breast segments.
3. Presentation: Cellulitis with fever, pain, redness, swelling, and malaise
4. Diagnosis/management
a. Antibiotics: Penicillinase-resistant cephalosporin
b. Continue breast feeding to help drain the engorged breast
c. Consider abscess drainage if infection persists
d. Consider biopsy if refractory to treatment to exclude malignancy
G. Mastitis in nonlactating women
1. Epidemiology: Most common in premenopausal women
a. Squamous epithelium extends abnormally into duct orifices trapping keratin, causing dilation and eventual rupture of ducts.
b. Recurrent infections associated with smoking secondary to promotion of squamous metaplasia of duct lining.
3. Presentation: Periareolar inflammation, may have purulent nipple discharge
4. Diagnosis/management
a. Antibiotics: Aerobic and anaerobic coverage
b. Aspiration if abscess present
c. Terminal duct excision for recurrent infections
d. Consider open drainage with biopsy if refractory to treatment
III. PROLIFERATIVE BENIGN BREAST DISEASE
A. Papilloma
1. *Epidemiology: Most common cause of bloody nipple discharge in women 20 to 40 years old.
2. Etiology: Intraductal epithelial tumor
3. Presentation: Spontaneous bloody, serous, or cloudy nipple discharge; typically not palpable
a. Nipple discharge considered to be pathologic if it is spontaneous, arises from a single duct, is persistent, and contains blood.
b. Discharge is physiologic if it occurs only in response to nipple compression, originates from multiple ducts, and often bilateral.
4. Diagnosis/management
a. Increased malignant potential after age 60 or with atypia
b. Mammography and ultrasound may reveal nonpalpable masses, calcifications, or dilated ducts.
c. Galactogram: Mammography performed after the offending lactiferous duct has been cannulated and filled with a contrast agent; localizes peripheral lesions
d. If biopsy reveals pathologic discharge, treat with terminal duct excision.
B. Phyllodes tumor
1. Epidemiology: Average age 45 years
2. Etiology
a. Rapid growth of a fibroepithelial periductal tumor
b. Malignant degeneration to sarcoma is reported in 6% of cases
3. Presentation: Very large, firm mass that is mobile and painless and may be difficult to distinguish from a fibroadenoma
a. Metastatic involvement of lymph nodes is rare, although patients may have palpable axillary lymphadenopathy
b. Metastases most frequently involve the lungs
4. Diagnosis/management
a. Core needle biopsy preferred method for diagnosis
b. Treat with wide local excision with >1 cm margins
c. If margins positive, patient should undergo reexcision to decrease the risk of local recurrence.
d. No axillary node dissection is required
e. Postoperative radiation and chemotherapy is controversial, but may be indicated for histologically malignant tumors or metastatic disease.
IV. PROLIFERATIVE BENIGN BREAST DISEASE WITH ATYPIA
A. Atypical ductal hyperplasia
1. Resembles low-grade ductal carcinoma in situ (DCIS)
2. Characterized by proliferation of uniform, evenly spaced epithelial cells with low-grade nuclei involving a limited extent of a duct.
B. Atypical lobular hyperplasia
1. Resembles lobular carcinoma in situ (LCIS) and is characterized by monomorphic, evenly spaced cells with a thin rim of clear cytoplasm.
2. Often containing clear vacuoles that involve <50% of the acini within a lobule.
C. Risk reduction strategies for high-risk women
1. Excision of abnormal cells versus prophylactic mastectomy
2. Annual surveillance mammograms
3. Discontinuation of oral contraceptives or HRT
4. Tamoxifen
PREMALIGNANT (NONINVASIVE) BREAST DISEASE
I. LCIS
A. Epidemiology: Premenopausal, ages 40 to 50
B. Etiology: >50% of acini are filled and distended with characteristic cells
C. Presentation
1. Usually incidental finding in women undergoing biopsy
2. Does not form a palpable mass
3. No specific mammographic finding
D. Prognosis: Regarded as a marker of increased risk rather than a true precursor
1. Relative risk of breast cancer is 5.4% to 12% (1% per year)
2. When invasive cancer does develop, it is more commonly ductal than lobular
3. Risk affects both breasts and persists indefinitely after diagnosis made
E. Diagnosis/management
1. Controversial, but includes lifelong surveillance with the goal of detecting subsequent malignancy at an early stage
2. Possible chemoprevention
3. Prophylactic bilateral mastectomy
II. DCIS OR INTRADUCTAL CARCINOMA
A. Epidemiology: Majority of in situ breast disease; risk increases with age
B. Etiology: Abnormal proliferation of epithelial cells confined within basement membrane.
C. Presentation
1. Usually first noted on mammography as clustered microcalcifications.
2. Less commonly may present as a palpable mass, pathologic nipple discharge, or Paget’s disease of the nipple.
D. Prognosis
1. Classified based on nuclear grade (low, intermediate, and high) and necrosis
2. About 50% of local recurrences after excision contain invasive carcinoma
3. Younger women are at higher risk for recurrence
E. Diagnosis/management
1. Mammography to evaluate the extent of calcifications
2. Diagnosis via image-guided core biopsy
3. Treated with breast-conserving therapy (BCT, lumpectomy + radiation) or mastectomy plus sentinel lymph node (SLN) biopsy
4. Adjuvant treatment with radiation therapy or endocrine therapy
III. PAGET DISEASE OF THE NIPPLE
A. Epidemiology: Rare, peak incidence ages 50 to 60
B. Etiology: Form of DCIS that spreads from ductal system into epidermis of nipple
C. Presentation
1. Scaly, ulcerated nipple associated with erythema, pain, and/or pruritus
2. Palpable breast mass in 50% of cases
D. Diagnosis/management: Diagnosed via full-thickness punch biopsy of nipple
1. Hallmark is the presence of malignant, intraepithelial adenocarcinoma cells (Paget’s cells) within epidermis of nipple
2. Treatment and prognosis depend on the stage of underlying carcinoma
MALIGNANT (INVASIVE) BREAST DISEASE
I. EPIDEMIOLOGY
A. The most common cancer in American women: Incidence, one-eighth
B. Second most common cause of death in American women (heart disease is first)
C. Risk factors
1. Age and ethnicity
a. Greatest risk after age 65
b. Before age 40, African-American women at higher risk; after age 40, Caucasian women at higher risk
2. Family history
a. About 20% to 30% of women with breast cancer have a positive family history, but only 5% to 10% have an inherited mutation in a breast cancer susceptibility gene.
b. *BRCA1 or BRCA2 mutations cause majority of hereditary breast cancer
i. Lifetime risk of breast cancer of up to 85%
ii. Increased risk of contralateral breast cancer and ovarian cancer (higher with BRCA1).
iii. BRCA2 increased the risk of male breast cancer, prostate cancer, and pancreatic cancer.
iv. Suspect hereditary breast cancer if 2+ first-degree relatives or 2+ generations with early-onset breast/ovarian cancer.
3. Hormonal factors: Risk increased with estrogen exposure
a. Menarche <12 years old
b. First pregnancy after age 35
c. Nulliparity
d. Increased age at menopause
e. Obesity
f. Estrogen replacement therapy >5 years
4. Environment and diet
a. Ionizing radiation (medical or nuclear) >90 rads
b. Increased alcohol intake
5. Other risk factors include a history of other neoplasms (contralateral breast, uterine or ovarian cancer, and major salivary gland carcinoma), atypical hyperplasia, and DCIS or LCIS
II. EVALUATION AND DIAGNOSIS
A. Diagnostic mammogram: Performed if abnormality detected on clinical examination or screening mammogram.
B. Ultrasound: Adjunct imaging study for women <40 years old and/or with palpable breast mass.
C. MRI: Indicated in some high-risk women and to evaluate for a primary breast tumor in patients presenting with axillary adenopathy.
D. Fine-needle aspiration: Minimally invasive way to obtain cells for diagnosis
E. Image-guided core biopsy: Core of tissue obtained for histologic examination
F. Excisional biopsy: Complete removal of breast mass, serves as definitive therapy for benign breast mass, and may be therapeutic lumpectomy if margins are negative.
G. Incisional biopsy: Reserved for masses too large to be completely excised
H. Wire-localization biopsy or lumpectomy: Lesion identified radiographically and marked with a wire prior to excision. After excision, specimen can be imaged to ensure removal of calcifications.
III. TYPES OF INVASIVE BREAST CANCER
A. Invasive cancer refers to cases in which tumor cells have crossed the basement membrane and have the ability to metastasize
B. Infiltrating ductal carcinoma
1. Most common invasive breast cancer (65% to 80%)
2. Presents as firm, fixed, irregular mass
C. Infiltrating lobular carcinoma
1. Grows as single file of malignant cells circumferentially arranged around ducts and lobules, and therefore does not usually produce distinct mass.
2. Accounts for 10% of breast cancer
D. Histologic subtypes
1. Tubular: Well differentiated, form normal appearing tubules, no myoepithelial cells; nodal metastases rare; excellent prognosis.
2. Mucinous/colloid: Large amount of extracellular mucus, relatively acellular; excellent prognosis
3. Medullary: Large, pleomorphic nuclei; high mitotic rate; grossly tumors are well circumscribed; nodal metastases unlikely; favorable prognosis
4. Cribriform: Well differentiated, gaps between cancer cells give a Swiss cheese appearance
5. Adenoid cystic: Rare subtype, cells resemble glandular and cystic cells
6. Juvenile secretory: Rare subtype, cells with clear vacuolated cytoplasm, most common type of breast cancer in children
IV. GRADING. Based on the degree of differentiation
A. Nuclear: Well differentiated, intermediate, or poorly differentiated
B. Histologic: Differentiation, growth pattern, extent of tubule formation, nuclear hyperchromasia, and mitotic index
V. HORMONE RECEPTORS
A. Estrogen and progesterone receptors (ER/PR) are nuclear hormone receptors that function as transcription factors when bound to their appropriate ligand.
B. Approximately 70% of breast cancers are ER/PR positive, and approximately 60% of those respond to endocrine therapy.
C. ER/PR positive tumors are candidates for hormonal therapy.
VI. STAGING
A. Refers to the grouping of patients according to the extent of their disease
B. Staging is critical for
1. Determining choice of treatment for a patient
2. Estimating prognosis
3. Comparing results of different treatment programs
C. Pathologic staging is the most precise estimator of prognosis and end result and can only be performed if tumor has negative surgical margins at the time of excision
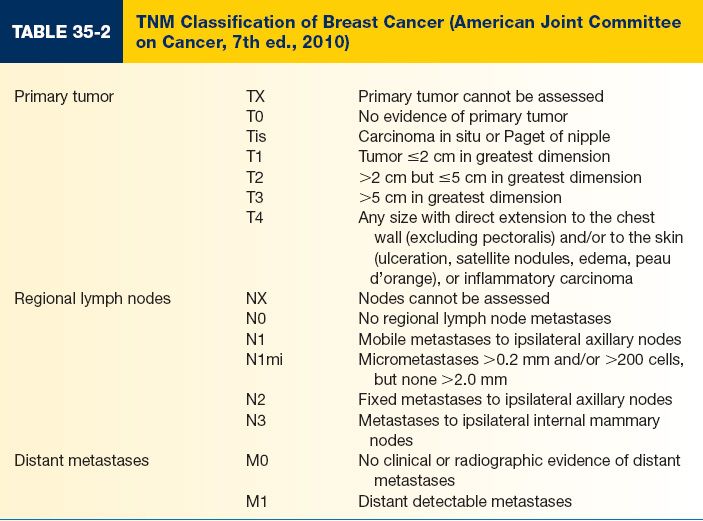
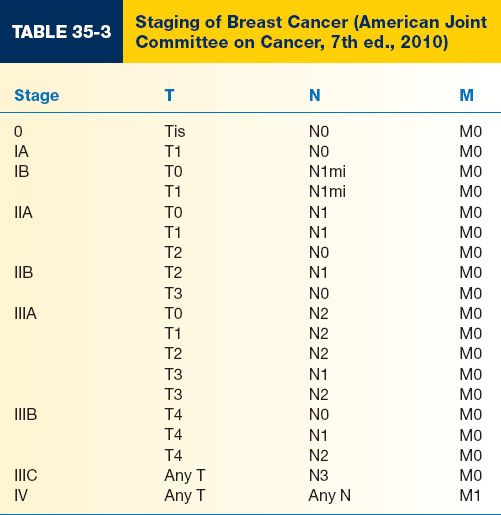
D. Determined by the American Joint Committee on Cancer (AJCC) using a system based on the TNM (tumor, node, metastasis) system (Tables 35-2 and 35-3)
VII. TREATMENT OF MALIGNANT BREAST DISEASE
A. Local therapy
1. BCT (Breast Conservation Therapy): Removal of primary tumor with adequate (usually 1 cm) margins of normal tissue and subsequent radiation therapy (XRT)
a. Indicated when it is possible to reduce the tumor burden to a microscopic level that can be controlled by radiation, radiation can be delivered safely, and local recurrence can be promptly detected.
b. Absolute contraindications
i. Two or more tumors in different quadrants
ii. Persistent positive margins after surgery
iii. First and second trimester of pregnancy. In third trimester, BCT can be performed and then XRT can be given after delivery.
iv. History of prior radiation to the breast
v. Diffuse microcalcifications with malignant characteristics
c. Neoadjuvant chemotherapy has not been shown to improve survival in BCT compared with postoperative therapy
2. Modified radical mastectomy (MRM)
a. Traditionally, MRM includes excision of the NAC
b. Nipple-sparing mastectomy is controversial
i. Indications: Select patients with clinically negative axillae, tumor >1 cm from NAC, and tumor size <3 cm
ii. Contraindications: Locally advanced breast cancer, inflammatory breast cancer
iii. Must perform intraoperative biopsy of retroareolar ducts and send for frozen section. If positive, must excise NAC.
c. Biopsy scar(s) should be included in skin excision
d. Skin flaps raised in the plane between the subcutaneous tissue and the underlying breast tissue.
e. Dissection should extend superiorly to clavicle, medially to sternum, inferi-orly to rectus sheath, and laterally to latissimus dorsi to ensure removal of all breast tissue.
f. Fascia overlying pectoralis major is considered the deep margin of resection, but can be preserved to facilitate reconstruction.
g. Breast flap viability should be assessed prior to placement of expander or implant. Adjunct perfusion scans (SPY) can be used to assess blood flow to flaps.
3. Multiple prospective, randomized trials with long-term follow-up have compared mastectomy with BCT: No demonstrated survival differences.
B. Axillary node management
1. Axillary lymph node dissection (ALND)
a. Level I and II: Standard practice; identifies metastases in 98% of patients
b. Level III: Performed only in patients with gross nodal metastases
2. Complications of ALND
a. Injury to axillary vein or motor nerves traveling within the axilla
b. *Lymphedema: Risk of development persists throughout a patient’s lifetime and increases as the time from surgery increases.
i. Highest risk following level III axillary dissections and axillary radiation.
c. Intercostobrachial nerve paresthesia (numbness on medial arm): 70% to 80% patients
d. Upper extremity pain and weakness: 20% to 30% after 1 year
3. SLN biopsy
a. The SLN is the first lymph node that receives drainage from a primary tumor.
i. Inject primary tumor with radioactive isotope-labeled colloid (i.e., technetium), lymphazurin blue dye, or both.
ii. SLN is detected via “hot” counts with a Geiger counter (radioactivity) or blue dye in node (lymphazurin).
iii. Remove all SLNs with counts >10% of the most radioactive node.
b. SLN can be identified in 90% of patients and predicts the status of the remaining axillary nodes in >90% of those patients.
c. Contraindications to SLN biopsy
i. Clinically suspicious axillary lymphadenopathy
ii. Inflammatory or other locally advanced cancer (T4 tumors)
iii. Pregnant or lactating woman
iv. Prior axillary surgery
C. Radiotherapy (XRT)
1. Indicated for BCT or 4+ positive lymph nodes
2. Reduces the risk of local recurrence
3. Improves survival by eradicating residual local disease that may be resistant to systemic chemotherapy
D. Adjuvant systemic therapy
1. Indicated to eliminate clinically occult micrometastases following local treatment of breast cancer to reduce recurrence and improve survival
2. Cytotoxic chemotherapeutic agents: Doxorubicin, cytoxan, methotrexate, 5-fluorouracil, and paclitaxel
a. *Selective estrogen receptor modulators, that is, tamoxifen: Has both estrogen antagonist and estrogen agonist properties.
i. Estrogen antagonist: Competitive blockade of estrogen receptors in breast.
ii. Estrogen agonist: Preserves bone density, lowers cholesterol levels, and increases the risk of endometrial carcinoma.
iii. Indicated for ER/PR positive tumors.
iv. Side effects include hot flashes, vaginal discharge, and increased risk of endometrial carcinoma and venous thromboembolism.
b. Aromatase inhibitor therapy, that is, anastrozole
i. Lowers estrogen levels by inhibiting the peripheral conversion of androgens to estrogen.
ii. Indicated for postmenopausal women.
iii. Side effect profile better than tamoxifen with fewer hot flashes, no endometrial effects, and fewer thromboembolic events.
iv. Higher risk of osteoporosis, fractures, and severe joint pain
SPECIAL CONSIDERATIONS
I. GYNECOMASTIA
A. Epidemiology: Peaks in incidence in infancy, puberty, and men ages 50 to 80
1. Overall incidence over 30%
2. Up to 65% of adolescent boys
B. Etiology
1. Proliferation of glandular tissue of male breast
2. Infancy: Transient gynecomastia secondary to high levels of maternal estrogen
a. Resolves 2 to 3 weeks after delivery
b. Most common hyperplastic childhood breast anomaly
3. Puberty: Onset between 10 and 12 years, spontaneously resolves within 6 months to 2 years of onset in most cases.
4. Medications and drugs
a. Spironolactone, digoxin, cimetidine, alcohol, ketoconazole, finasteride, and tricyclic antidepressants
b. HAART (highly active antiretroviral therapy) for human immunodeficiency virus/AIDS (acquired immunodeficiency syndrome) treatment
c. Anabolic steroids
d. Alcohol
e. Marijuana
f. Heroin
5. Cirrhosis
6. Male hypogonadism: Results in estrogen/androgen imbalance
a. Primary hypogonadism due to congenital abnormality such as Klinefelter syndrome.
b. Secondary hypogonadism due to a hypothalamic or pituitary abnormality.
c. Hyperprolactinemia: Prolactin reduces the secretion of gonadotropins
7. Testicular neoplasm: Germ cell, Leydig cell, or Sertoli cell tumors. *All males presenting for the evaluation of gynecomastia must have a testicular examination.
8. Hyperthyroidism: Due to Graves’ disease
9. Pseudogynecomastia: Often seen in obese males, refers to fat deposition without glandular proliferation.
10. Pneumonic: SACKED
a. S = spironolactone
b. A = alcohol, age, alopecia medications, antidepressants
c. C = cimetidine, cirrhosis
d. K = ketoconazole, Kleinfelter syndrome
e. E = excessive estrogen
f. D = digoxin, drugs
1. Unilateral or bilateral
2. Begins with subareolar enlargement
D. Physical examination
1. Breast
a. Assess the amount of adipose tissue versus glandular tissue
b. Ptosis
c. Skin excess
d. Masses
2. Testicular examination
3. Feminizing characteristics
4. Mass of thyroid, liver, or abdomen
E. Staging
1. Grade I: Minimal hypertrophy (<250 g) and no ptosis
2. Grade II: Moderate hypertrophy (250 to 500 g) and no ptosis
3. Grade III: Severe hypertrophy (>500 g) with grade I ptosis
4. Grade IV: Severe hypertrophy with grade II or III ptosis
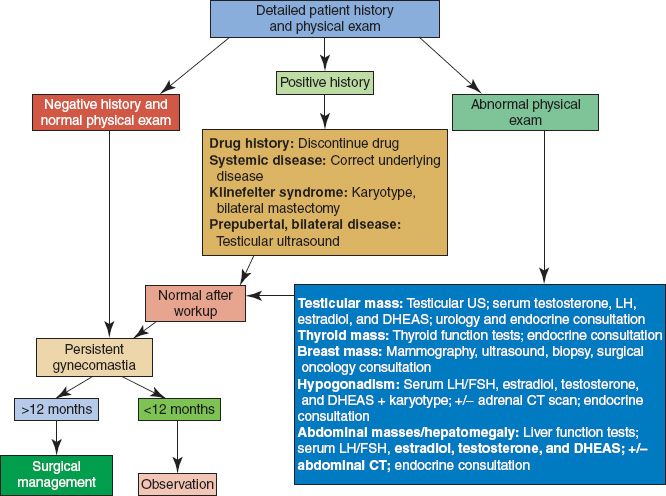
F. Diagnosis/management (Fig. 35-2)
1. Must rule out male breast cancer if asymmetric. If concerning, order a mammogram.
2. If persistent, painful, and/or no clear physiologic etiology, check labs: liver function tests, TSH, luteinizing hormone, follicle stimulating hormone, human chorionic gonadotrophin, prolactin, estradiol, testosterone, and androstenedione
3. Surgical treatment depends on the severity of disease
a. Excess fat with minimal excess skin/gland: Liposuction only
b. Subareolar glandular tissue with minimal excess skin: Liposuction + periareolar gland excision
c. Excess skin, fat, and gland: Inframammary incision with free nipple graft
Figure 35-2. Gynecomastia workup algorithm. DHEAS, dehydroepiandrosterone-sulfate; LH/FSH, luteinizing hormone/follicle-stimulating hormone; US, ultrasound; CT, computed tomography.
A. Risk factors
1. Positive family history, BRCA2 mutation, and radiation exposure.
2. Hormonal imbalance leading to high ratio of estrogen-to-androgen, secondary to conditions such as cirrhosis and Klinefelter syndrome.
3. No clear association with gynecomastia except with Klinefelter syndrome.
B. Epidemiology
1. Mean age of 65 to 70 years
2. 1% of all breast cancer cases
C. Presentation
1. Mass beneath the NAC associated with retraction or ulceration of nipple.
2. Differential diagnosis for a breast mass in a male includes gynecomastia, abscess, or metastases to the breast from remote primary tumor (i.e., sarcoma).
D. Diagnosis/management
1. Same diagnostic algorithm as female breast cancer.
2. Mastectomy is the most common treatment
a. Total mastectomy if limited pectoral muscle involvement.
b. Radical mastectomy if extensive pectoral muscle invasion present.
3. Hormonal therapy: Most beneficial if the tumor is hormone receptor positive
a. Approximately 80% of male breast cancers are hormone receptor positive.
b. Tamoxifen: Improves survival rates, but may not be well tolerated in men and may cause weight gain and decreased libido.
c. Orchiectomy: Reserved for patients with metastatic disease refractory to other medical treatments.
E. Prognosis: When matched by stage, survival is similar to women with breast cancer. Axillary nodal status is the major predictor of outcome.
PEARLS
1. The intercostobrachial nerve is a cutaneous nerve that transversely crosses the axilla to supply the skin of the medial aspect of the upper arm. Injury to this nerve during an ALND causes numbness of this region.
2. Depending on surgeon preference, patients may need to discontinue hormonal therapy prior to major surgeries
3. A complete workup for gynecomastia always includes a testicular examination and review of medications
QUESTIONS YOU WILL BE ASKED
1. What is the differential diagnosis for a breast mass?
Cyst, fibroadenoma, fibrocystic changes, fat necrosis, and cancer.
2. What is the most common cause of bloody nipple discharge in women 20 to 40 years old?
Papilloma.
3. What are the anatomic boundaries of the breast?
Clavicle to sixth rib, sternal edge to midaxillary line, and posteriorly the fascia of pectoralis major and serratus anterior.
4. What is the dominant blood supply to the breast?
Internal mammary artery.
5. What is the innervation of the nipple–areolar complex?
Fourth intercostal nerve.
Recommended Readings
Chang DS, McGrath MH. Management of benign tumors of the adolescent breast. Plast Reconstr Surg. 2007;120(1):13e–19e. PMID: 17572540.
Howard JH, Bland KI. Current management and treatment strategies for breast cancer. Curr Opin Obstet Gynecol. 2012;24(1):44–48. PMID: 22123219.
Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275–285. PMID: 16034013.
< div class='tao-gold-member'>