Breast Cancer Diagnosis, Prognosis, and Treatment in Augmented Women
Neal Handel
History
Over the years concerns have been raised about the possible effects of silicone implants on the incidence, detection, treatment, and prognosis of breast cancer. Breast augmentation is a commonly performed elective procedure, making these concerns legitimate. Recent statistics published by the American Society of Plastic Surgery (ASPS) indicate breast augmentation is the most popular cosmetic surgical procedure in the United States. Nearly 314,000 primary breast augmentations were performed in 2018, up 4% from 2017 (1).
Carcinoma of the breast is also common (2). The American Cancer Society estimates that women in the United States have nearly a 1 in 7 (13.4%) lifetime risk of developing breast cancer (3). In 2019, an estimated 268,600 women in the United States will be diagnosed with invasive breast cancer, and 62,930 women will be diagnosed with in situ breast cancer.
It is estimated that 42,260 deaths from breast cancer will occur this year (4). Extrapolating from these statistics, more than 42,000 augmented women will be diagnosed annually with breast cancer. It is therefore important to understand the potential effects of implants on the incidence, detection, treatment, and prognosis of breast cancer.
TABLE 15-1 Incidence of Breast Cancer in Augmented Patients | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Implants and Cancer Risk
There have long been questions about a possible association between implants and the risk of developing carcinoma of the breast or other benign or malignant breast tumors. The origin of these concerns was based on the observation that when solid foreign bodies made of various different substances were implanted in rodents, they frequently elicited the development of sarcomas (5,6). This phenomenon, known as the Oppenheimer effect, has never been observed in humans (7,8,9). Numerous epidemiologic studies have investigated the incidence of breast cancer in augmented patients compared to nonaugmented cohorts. These studies unequivocally demonstrate that breast implants are not associated with an increased risk of developing carcinomas or other types of benign or malignant breast tumors (10,11,12). In fact, recently published studies (13,14,15,16) have documented a lower-than-expected incidence of breast cancer in augmented patients (Table 15-1). The observation that augmented women appear to be at a somewhat lower risk of developing breast cancer has led to speculation about possible mechanisms whereby breast implants might inhibit tumorigenesis. Among the hypotheses that have been suggested are that implants cause a heightened immune response, leading to earlier detection and
destruction of precancerous cells; that the compression effect of the implant on surrounding breast tissue results in an alteration of cell growth rate; and that the implant acts as insulation, lowering the ambient temperature of the breast, with subsequent reduction of local tissue metabolic rates (13).
destruction of precancerous cells; that the compression effect of the implant on surrounding breast tissue results in an alteration of cell growth rate; and that the implant acts as insulation, lowering the ambient temperature of the breast, with subsequent reduction of local tissue metabolic rates (13).
While it clearly has been established that augmented women are not at increased risk for developing breast cancer, due to the frequency of this disease, many augmented women eventually will be diagnosed with breast cancer. This has resulted in persistent and valid concerns about the possible effects of implants on cancer detection (22,23). Conceivably, implants could alter physical examination of the breast, reduce the sensitivity of various imaging modalities, or even interfere with adequate biopsy of suspicious lesions.
Physical Examination of the Augmented Breast
Breasts implants, whether submammary, submuscular, or in a dual plane pocket, always reside beneath the parenchyma. For that reason, the presence of the prosthesis alone should not compromise palpation of a lesion. However, there are numerous ways implants may alter physical examination of the breast. Sometimes breast augmentation (particularly when combined with mastopexy) causes parenchymal scarring or areas of fat necrosis. This may result in thickened regions or even discrete lumps palpable on physical examination. If such findings are clinically suspicious, further diagnostic testing is indicated.
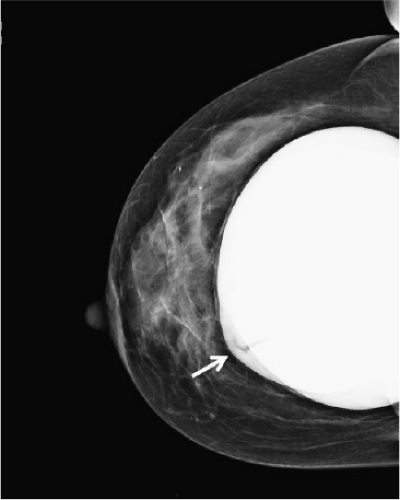
A significant number of augmented patients develop capsular contracture. Ordinarily contracture does not interfere with palpation of breast tissue, but occasionally the capsule tears, allowing part of the implant to herniate into adjacent parenchyma; this may result in a palpable (or even visible) mass within the breast (Fig. 15-1). Typically, such a palpable bulge or “knuckle” of the implant is not confusing to plastic surgeons. However, primary care physicians, gynecologists, and nurse practitioners may not be familiar with these findings; when such an abnormal mass is identified, it needs to be differentiated from a pathologic lesion. Calcification of the capsule sometimes occurs, and rigid calcium deposits may be palpated immediately adjacent to the implant. These benign calcifications must be distinguished on both physical examination and mammography from malignant microcalcifications (Fig. 15-2A,B).
 FIGURE 15-1 Augmented patient with a herniated silicone gel breast implant presenting as a palpable and visible breast mass. |
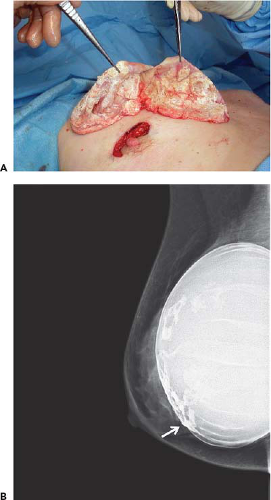 FIGURE 15-2 A: Dense calcification of scar tissue capsule surrounding silicone gel implant. B: Digital mammogram showing diffuse “egg shell” calcifications within the capsule surrounding the implant. |
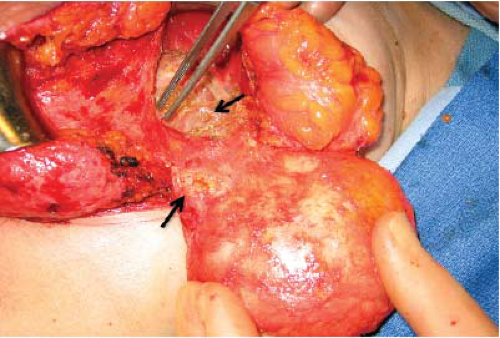 FIGURE 15-3 Multiple siliconomas in breast parenchyma and adjacent soft tissues in a patient with a ruptured silicone gel implant. |
One of the recognized complications of silicone gel implants is leakage of filler material, from either gel bleed or frank rupture of the elastomeric shell. When silicone gel leaks from the implant, it may elicit an inflammatory foreign body reaction, leading to fibrosis and sometimes formation of siliconomas. These abnormalities may be palpable adjacent to the implant or in other areas of the breast or chest wall (Fig. 15-3), depending upon where the silicone migrates. Siliconomas typically present as firm, discrete lumps and must be differentiated from other pathologic masses. If a silicone implant ruptures and there is spread of silicone into the breast parenchyma or adjacent structures (extracapsular rupture), it is readily apparent on mammograms (Fig. 15-4). When the silicone gel leaking from the implant remains intracapsular, it may be difficult or impossible to detect on physical examination (silent rupture). This is especially true with newer-generation, more highly cohesive gel-filled implants. Intracapsular rupture can sometimes be diagnosed on mammography (Fig. 15-5) and can reliably be imaged by magnetic resonance imaging (MRI).
Saline implants are inflated with a fill tube inserted via a self-sealing valve on the anterior or posterior surface of the implant. In some patients, particularly those with a thin layer of breast tissue, the valve may be palpable. Generally it will be felt in the central portion of the breast, beneath the nipple areolar complex. Even in cases in which the valve is on the posterior surface, the device can flip over and the valve may be felt. Surgeons sometimes unwittingly perform biopsies only to discover that the palpable mass is a filler valve.
When performing surgical or needle biopsy of a suspected abnormality, caution must be exercised to avoid damaging the implant. In the case of fine-needle aspiration or core needle biopsy, the implant can usually be manipulated away from the area where the sharp
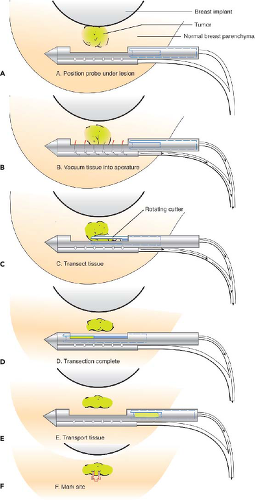
tip is introduced. However, if the lesion is immediately adjacent to the implant, it may be preferable to perform open biopsy to avoid damaging the prosthesis (Fig. 15-6). Core needle biopsy can be problematic because multiple needle insertions may be required to obtain adequate tissue samples, increasing the risk of puncturing the prosthesis. Vacuum-assisted biopsy (Mammotome, MIBB) facilitates biopsy in women with implants (Fig. 15-7) (24,25). With this technique, stereotactic mammography or real-time ultrasonography is used to guide the needle tip adjacent to the suspicious area. The vacuum-assisted biopsy needle can be accurately positioned near the lesion to avoid damaging the implant. Unlike core needle biopsy, the vacuum-assisted biopsy probe is inserted just once into the breast through a single small skin incision. Multiple tissue samples may be taken by rotating the needle aperture and using vacuum assistance. The precision and directional capabilities of vacuum-assisted biopsy make this the preferred technique for percutaneous biopsy in augmented women.
tip is introduced. However, if the lesion is immediately adjacent to the implant, it may be preferable to perform open biopsy to avoid damaging the prosthesis (Fig. 15-6). Core needle biopsy can be problematic because multiple needle insertions may be required to obtain adequate tissue samples, increasing the risk of puncturing the prosthesis. Vacuum-assisted biopsy (Mammotome, MIBB) facilitates biopsy in women with implants (Fig. 15-7) (24,25). With this technique, stereotactic mammography or real-time ultrasonography is used to guide the needle tip adjacent to the suspicious area. The vacuum-assisted biopsy needle can be accurately positioned near the lesion to avoid damaging the implant. Unlike core needle biopsy, the vacuum-assisted biopsy probe is inserted just once into the breast through a single small skin incision. Multiple tissue samples may be taken by rotating the needle aperture and using vacuum assistance. The precision and directional capabilities of vacuum-assisted biopsy make this the preferred technique for percutaneous biopsy in augmented women.
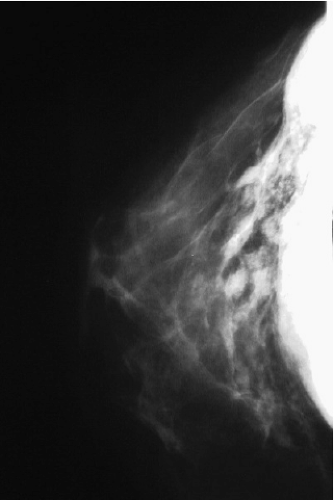 FIGURE 15-4 Mammogram of a patient with extracapsular rupture of a silicone gel implant, showing extravasation of free silicone into the ducts and breast parenchyma. |
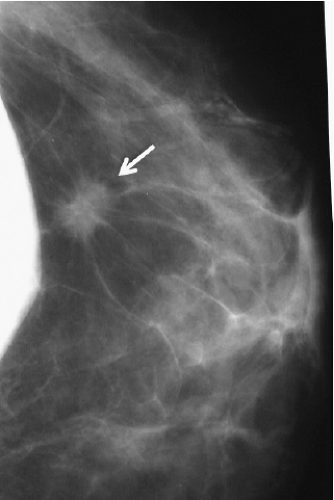 FIGURE 15-6 Mammogram of augmented patient with a breast cancer in close proximity to breast implant. |
When open biopsy is performed, steps can be taken to minimize the risk of damaging the implant. Dissection is best performed with a blunt-tipped electrocautery. If the surface of the implant is encountered, care should be taken to avoid damaging the shell with any pointed or sharp instrument. If the surgical biopsy also includes a portion of the capsule, it is unnecessary to repair the capsule; a new layer of scar tissue will regenerate over the implant surface.
Radiologic Imaging of the Augmented Breast
Mammography
Routine mammographic screening of asymptomatic women facilitates early diagnosis of breast cancer. Over the years concerns have been raised about the accuracy of mammography in augmented patients (26,27). There are several ways implants may affect mammograms. The surgery can cause parenchymal scarring, resulting in architectural distortions, densities, or calcifications that appear on mammography. Implants (particularly when present over a prolonged period of time) compress breast tissue, increasing radiodensity, reducing contrast, and potentially interfering with identification of subtle lesions (28). The best-quality mammograms are obtained when the breast is maximally compressed so the x-ray beam penetrates the thinnest possible layer of tissue. With less compression, the volume of visualized tissue per unit area increases, causing more superimposition and potentially reducing mammographic sensitivity. Because implants are less compliant than breast tissue, they make it difficult to achieve the desired compression. During mammography the average nonaugmented breast can be compressed to a thickness of 4.5 cm, while the average augmented breast can be compressed only to a thickness of 7 cm (29).
The most important factor, affecting mammography in the augmented breast is the radiopaque shadow cast by the implant. Early reports estimated that only 25% of the breast is visualized after augmentation (30). Gumucio et al. (31) demonstrated that both saline and silicone implants can totally obscure early lesions such as microcalcifications. Hayes et al. (32) reported that 22% to 83% (38% on average) of the breast tissue could be obscured by the implant.
The extent to which an implant shadow interferes with mammography depends upon its size (33) and radiodensity (34). The density of the shadow is determined primarily by the physical and radiologic characteristics of the filler material. Both silicone and saline create a dense, radiopaque shadow that completely blocks visualization of adjacent breast tissue. Over the years several prostheses (e.g., Misti Gold implant, Trilucent implant) containing alternative filler materials have been introduced. However, none of these fillers proved entirely satisfactory and at the present time only saline- and silicone gel–filled implants are commercially available.
Prior studies have investigated how breast implants affect the amount of tissue visualized on mammograms (27,34). Preoperative and postoperative mammography was performed in a consecutive series of breast
augmentation patients, and the amount of tissue visualized on each film was measured. Changes in area visualized were correlated with various parameters, including the degree of capsular contracture, implant position (submammary vs. submuscular), type of mammography (compression vs. displacement), preoperative breast size, implant size, and implant type.
augmentation patients, and the amount of tissue visualized on each film was measured. Changes in area visualized were correlated with various parameters, including the degree of capsular contracture, implant position (submammary vs. submuscular), type of mammography (compression vs. displacement), preoperative breast size, implant size, and implant type.
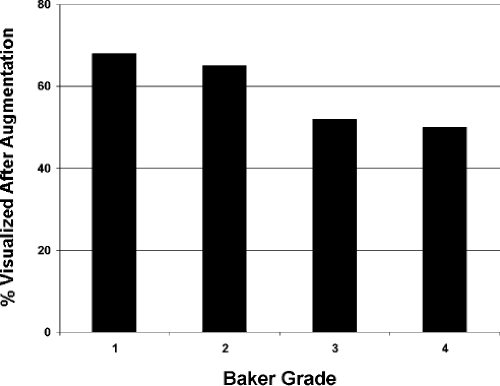
Our study revealed that in most patients there is a decrease in measurable breast tissue on postaugmentation films. The most important factor is capsular contracture: Little or no contracture (Baker 1 or 2) resulted in a 30% reduction in the area visualized, while moderate or severe contracture (Baker 3 or 4) resulted in a 50% reduction (Fig. 15-8). In patients who had serial mammography in the face of worsening contracture, there was progressive reduction in the amount of tissue visualized (Fig. 15-9). Implant position (submammary vs. submuscular) also proved important; on average, patients with submammary implants had a 37% reduction in area visualized, while patients with submuscular implants had only a 17% reduction.
In order to facilitate mammography in augmented women, Eklund et al. (35) developed the displacement or “push-back” technique (Fig. 15-10A,B), by which the implant is displaced posteriorly to allow a greater proportion of breast tissue to be captured on the mammogram. Using standard compression mammography, Eklund et al. determined that up to 97% of the breast tissue could be obscured by the implant, greatly increasing the likelihood of missing a significant lesion. The displacement technique led to an improvement in 99% of cases. Part of this improvement is likely attributable to the fact that implant displacement results in a compression advantage of up to 5 cm, leading to improved image quality and greater sensitivity. Subsequent studies confirmed the results of Eklund et al. in obtaining better visualization of breast tissue using implant displacement (36,37). Our study (38) also confirmed that slightly more tissue is visualized with displacement (average reduction of 25%) than with standard compression mammography (average decrease of 30%); however, with either technique there is a substantial reduction in area visualized compared to preaugmentation mammograms (Fig. 15-11).
While no one refutes that the shadow cast by an implant interferes with visualization of breast tissue, there has been debate about whether this translates into diminished mammographic sensitivity. It has been
established that mammography in nonaugmented women is highly sensitive. In nonaugmented women with palpable tumors, the mammogram is positive in >90% and the false-negative rate is <10%. There are several published reports suggesting that the false-negative rate in augmented patients is considerably higher (37,39,40). We recently reviewed the mammograms of all patients with palpable tumors treated over a 23-year period (41). Mammograms among 1,741 nonaugmented women failed to visualize the tumor in 153 (false-negative rate of 8.8%). The mammograms of 87 augmented patients failed to reveal the lesion in 36 cases (false-negative rate of 41.4%). This difference is highly significant (p < 0.0001) (Table 15-2) and suggests that implants dramatically reduce the sensitivity of mammography.
established that mammography in nonaugmented women is highly sensitive. In nonaugmented women with palpable tumors, the mammogram is positive in >90% and the false-negative rate is <10%. There are several published reports suggesting that the false-negative rate in augmented patients is considerably higher (37,39,40). We recently reviewed the mammograms of all patients with palpable tumors treated over a 23-year period (41). Mammograms among 1,741 nonaugmented women failed to visualize the tumor in 153 (false-negative rate of 8.8%). The mammograms of 87 augmented patients failed to reveal the lesion in 36 cases (false-negative rate of 41.4%). This difference is highly significant (p < 0.0001) (Table 15-2) and suggests that implants dramatically reduce the sensitivity of mammography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree