Breast Cancer: Current Trends in Screening, Patient Evaluation, and Treatment
Grant W. Carlson
Cancer of the breast is the most common cancer in women with the exception of skin cancer. It is the second leading cause of cancer death after lung cancer. Approximately 200,000 new cases of breast cancer are diagnosed each year in the United States. They account for over 40,000 deaths a year. The incidence of breast cancer has decreased over the last decade largely due to a discontinuation of hormone replacement therapy among postmenopausal women.
The treatment of breast cancer has evolved because of the results of large, prospective, randomized clinical trials organized by the National Surgical Adjunctive Breast and Bowel Project (NSABP) in the United States and the National Cancer Institute in Milan, Italy. The majority of women with breast cancer are eligible for breast conservation therapy and receive some form of systemic adjuvant therapy.
RISK FACTORS
One in nine women in the United States who reaches the age of 85 will develop breast cancer. The etiology is unknown but is clearly multifactorial, with many exogenous and endogenous risk factors being identified (Table 58.1).
Aside from gender, age is the single most important factor in determining breast cancer risk. The probability that breast cancer will develop increases throughout a woman’s life, with half of all cases occurring in women older than age 65. Family history is also important since 20% of breast cancer patients will have a relative with the disease. The magnitude of breast cancer risk is influenced by several factors pertaining to family history: number and proximity of affected relatives, their menstrual status, age at diagnosis, and the presence of bilateral cancer.
Hereditary breast cancer accounts for 5% to 10% of breast cancer cases and is caused largely by the presence of BRCA gene mutations. The genes are more common in women of Ashkenazi ancestry, patients with bilateral breast cancer, cancer diagnosed before age 50, and patients with ovarian cancer. The presence of a BRCA gene confers a 60% to 85% risk of developing breast cancer and a 10% to 40% risk of developing ovarian cancer.
TABLE 58.1 BREAST CANCER RISK FACTORS | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
Many epidemiological studies have linked early menarche, late menopause, and late age at first full-term pregnancy to breast cancer. The total duration of menstrual cycles and the number of menstrual cycles before full-term pregnancy appear to be proportional to breast cancer risk. Premalignant histology on breast biopsy may increase breast cancer risk, as discussed in the following section. A woman with unilateral breast cancer is at increased risk for developing cancer in the opposite breast. Studies have not shown that the development of contralateral breast cancer impacts adversely on survival.
PATHOLOGY
Screening mammography, by detecting early breast cancers, has increased our understanding of the malignant transformation process. Most cancers arise from the ductal elements of the breast after passing, presumably, through a sequence of premalignant stages as depicted below.
Normal breast → hyperplasia → atypical hyperplasia → ductal carcinoma in situ → invasive cancer
This process can occur over a 10- to 20-year period, and orderly progression through the various stages is not obligatory. Ductal carcinoma in situ (DCIS), also known as intraductal carcinoma, is cancer confined by the basement membrane of the ducts. DCIS most commonly presents as mammographic microcalcifications and currently comprises 30% of newly diagnosed cancers in populations following screening mammography guidelines. DCIS occurs in several histological patterns with varying propensities to progress to invasive cancer. Comedo DCIS is characterized by pleomorphic cells, high-grade nuclei, and central areas of necrosis. Noncomedo DCIS occurs in several subtypes that are generally not as cytologically malignant as comedo DCIS. It may be difficult to distinguish noncomedo DCIS from atypical hyperplasia.
Invasive ductal carcinoma accounts for the majority of breast cancer cases. Grossly, it appears as a gray-white, irregular, speculated mass that is hard and gritty on cut section. It has no specific microscopic features but can be recognized histologically as an invasive adenocarcinoma involving the ductal elements.
A number of histological variants arise from ductal epithelium. Medullary carcinoma is grossly soft and fleshy and accounts for 6% of invasive cancers. It tends to grow to a large size and is well circumscribed. Histologically, it is characterized by poorly differentiated nuclei and infiltration by lymphocytes. Medullary carcinoma has a favorable prognosis even in the presence of nodal metastases. Tubular carcinoma is a rare histological variant in its pure form and accounts for 2% of breast cancer. It is characteristically small and is
usually found on mammography. It tends to be highly differentiated and has an excellent prognosis. Mucinous or colloid carcinoma is another well-differentiated variant, which tends to form a well-circumscribed soft, gelatinous mass. Histologically, nests of tumor cells are surrounded by a mucinous matrix.
usually found on mammography. It tends to be highly differentiated and has an excellent prognosis. Mucinous or colloid carcinoma is another well-differentiated variant, which tends to form a well-circumscribed soft, gelatinous mass. Histologically, nests of tumor cells are surrounded by a mucinous matrix.
Although most cancers arise from the ductal elements, malignancies may also arise from the epithelium of the breast lobules. Lobular carcinoma in situ (LCIS) has no radiological or physical manifestations and has traditionally not been regarded as a malignancy. LCIS is usually an incidental finding after a biopsy of a mass or mammographic abnormality. Current evidence suggests that the histological diagnosis of LCIS confers a 20% risk of developing cancer in either breast at 20-year follow-up.
About 5% to 15% of infiltrating cancers arise from the breast lobules. Once it has become invasive, lobular carcinoma has a prognosis similar to the ductal type. It tends to be extensively infiltrative without a distinct tumor mass. Histologically, the cells demonstrate a characteristic single file pattern. The tumor does not form microcalcifications, and mammographic detection may be difficult.
SCREENING
The American Cancer Society recommends that women at average risk should begin annual mammography at the age of 40 years.1 This has been shown to reduce the risk of dying of breast cancer. Early detection can also result in less aggressive surgery and adjuvant therapy to treat the cancer. The sensitivity of mammography is related to patient age, breast density, and breast cancer histology. False-positive exams may result in additional breast biopsies, especially in young patients. There is no specific age to discontinue screening mammography.
Breast magnetic resonance imaging (MRI) is increasingly used for breast cancer screening. It has the greatest sensitivity of all imaging modalities for detecting breast cancer of a few millimeters in diameter. MRI suffers from a low specificity, which can result in unnecessary biopsies as well as a high cost. The American Cancer Society recommends breast MRI in the management of women at high risk for developing breast cancer beginning at age 30.2 This includes women with known or suspected BRCA gene mutations and women who have undergone mantle radiation to the chest for the treatment of Hodgkin’s disease. Women felt to have a 20% to 25% or greater lifetime risk of breast cancer based on risk estimation models are also included in this high-risk category
STAGING
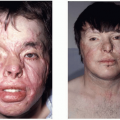
The American Joint Committee on Cancer TNM staging system is based on clinical as well as pathological information. The classification by primary tumor (T), status of axillary lymph nodes (N), and presence of distant metastases (M) places patients in different prognostic groups (Tables 58.2 and 58.3). Stages I and II are considered early breast cancer for which surgery plays a primary role in treatment. Stage III disease is also known as locally advanced breast cancer (LABC). Despite the absence of metastatic disease, this stage has a poor prognosis and is best treated with a combination of chemotherapy, surgery, and radiation therapy. This stage includes inflammatory breast cancer, a clinical entity characterized by breast warmth, erythema, and edema. The orange peel appearance of the skin, peau d’orange, results from dermal lymphatic invasion.
PATIENT EVALUATION
Most patients with breast cancer are diagnosed with imageguided core biopsy. Ultrasound of the axilla is routinely performed to screen for potential nodal spread. Mammography/ultrasound and histological information are used to guide patient management. Breast MRI is used in patients with dense breasts and in those presenting with lobular carcinoma to define the extent of disease. The modality is also useful in the detection of contralateral disease and the assessment of tumor response after neoadjuvant chemotherapy. There are concerns that breast MRI may overestimate the extent of disease, resulting in more patients being treated by total mastectomy.
LOCOREGIONAL TREATMENT
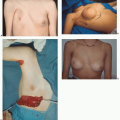
The goals of locoregional treatment are to provide optimal local control, adequate disease staging, long-term survival, and preservation or restoration of body form. Total mastectomy and axillary dissection were the standard treatment for over 50 years, based on the Halsted mechanistic theory of cancer dissemination. Halsted believed that cancer was predominantly a local disease that spreads by permeation of lymphatic pathways. He proposed the radical mastectomy to remove the cancer and prevent systemic spread. Numerous prospective randomized trials have refuted this theory of tumor biology. The bloodstream is an important pathway in early tumor dissemination, and more conservative locoregional treatment combined with systemic therapy has proved to provide local disease control with prolonged survival.
Breast Conservation
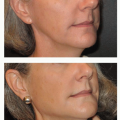
Breast conservation is the treatment of choice for the majority of stage I and II breast cancers. Six prospective randomized trials of over 4,300 women have found breast-conserving treatment to result in survival rates similar to those achieved by total mastectomy. Removal of the cancer with pathologically negative margins is termed as lumpectomy or partial mastectomy. The remaining breast is usually treated with 50 Gy of external breast radiation to improve local control. The NSABP B-06 trial compared total mastectomy, lumpectomy, and lumpectomy and radiation in 1,843 women.3 The survival was the same for all three groups but the addition of breast irradiation to lumpectomy reduced the local recurrence from 40% to 8%. Young patients and those with extensive intraductal cancer surrounding the invasive component are at increased risk for local recurrence. Because of the propensity for ductal carcinoma to spread upward toward the nipple along the duct, a quadrantectomy has been proposed to reduce local recurrence. Larger excisions result in slightly improved local control rates at the expense of the cosmetic result but have no impact on ultimate survival. Local recurrences are generally treated by total mastectomy.
There are few absolute contraindications to breast conservation (Table 58.4). The cosmetic outcome of lumpectomy is dependent on both treatment-related factors and patient selection and is judged to be excellent to good by 60% to 90% of patients.
Accelerated Partial Breast Irradiation
Whole breast irradiation after lumpectomy or partial mastectomy is the standard of care to prevent local recurrences. Studies have shown that most recurrences after breast conservation occur near the original disease site. Recurrence rates away from the tumor bed are similar after lumpectomy whether adjuvant whole breast irradiation is administered or not.4 This is the rationale for accelerated partial breast irradiation (APBI). It concentrates radiation to a partial volume of the breast over a 1- to 2-week period compared with a 6- to 7-week period for conventional whole breast irradiation. Potential advantages of APBI include convenience and less toxicity with the potential for better cosmetic results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree