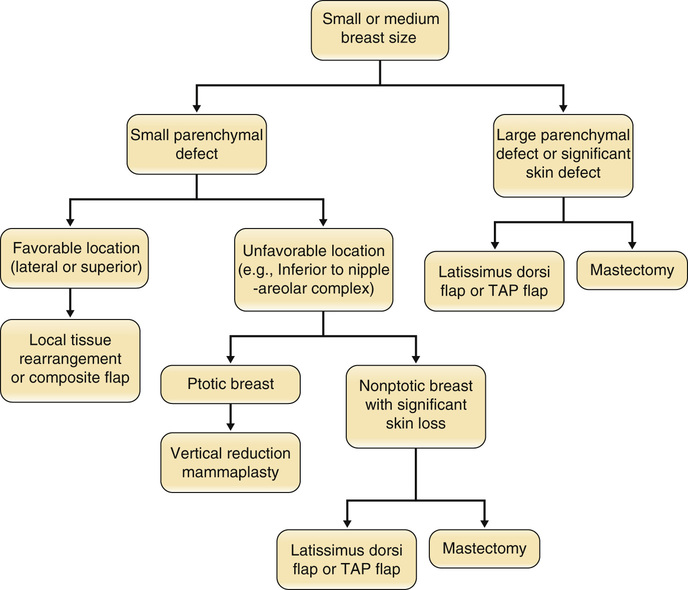
• Most common noncutaneous malignancy among women ○ First full-term pregnancy after 30 years of age (yo) ▪ Personal history of breast cancer or benign proliferative breast disease ▪ BRCA mutation (occurs in <1% of women; accounts for 5% of breast cancers; 60% to 80% lifetime risk) ▪ Breast cancer in a first-degree relative ○ Age of affected <50 yo → Risk ratio (RR) 2.3 ○ Age of affected >50 yo → RR 1.8 ○ Bilateral breast cancer → RR 5.5 ▪ Radiographic imaging of the breast that is an excellent screening tool for abnormal masses or calcifications ○ Linear or branching calcifications may suggest malignancy. ○ Pleomorphic/granular calcifications may suggest malignancy. ○ Popcorn-like calcifications suggest fibroadenoma. ○ Large rod-like calcifications suggest secretory ducts. ○ Round eggshell calcifications suggest oil cysts. ○ Dystrophic/coarse calcifications suggest fat necrosis. ◆ Calcifications can be found in ~25% of women who have had inferior pedicle breast reductions. ▪ Patients with implants may get mammograms; however, they require a special view called the Eklund view, where the prosthesis is pushed against the chest wall and the breast parenchyma is pulled anteriorly around and away from the implant. ○ Implant position, capsular contracture, and small native breasts can compromise the reliability of mammography. ◆ Implant size has not been shown to affect mammography. • Magnetic resonance imaging (MRI) ▪ Newer tool for evaluating the breast for breast cancer ▪ Especially useful for screening high-risk patients ▪ Widely used as a diagnostic modality; often used as a secondary study to work up a mass ▪ Useful in women with dense breasts (i.e., younger women) ▪ Ultrasound findings can help differentiate between malignant and benign lesions. • Histologic diagnostic modalities ◆ Does not provide structural information to determine invasive from in situ ◆ High false-negative rates from sampling error (2% to 22%) • Ductal carcinoma in situ (DCIS) • Lobular carcinoma in situ (LCIS) • Invasive ductal carcinoma (IDC) • Invasive lobular carcinoma (ILC) • Inflammatory breast carcinoma ▪ Can often involve chest wall, requiring a modified radical mastectomy with wide skin defect 4. Breast cancer staging (see Tables 9.1 and 9.2) Table 9.1 Staging of Breast Cancer: TNM System Table 9.2 TNM Stage and Survival ○ Equivalent to mastectomy in long-term survival ○ Requires adjunctive radiation to limit risk for local recurrence ◆ Radiation-related complications ◆ Unfavorable esthetic outcomes (estimated to be 20% to 35%) ○ Reconstructive options (see Figures 9.1 and 9.2) ◆ Oncoplastic reduction or mastopexy ➔ Balancing the resection with breast landmarks and shape to preserve aesthetics ◆ Lat flap
Breast Cancer and Breast Reconstruction
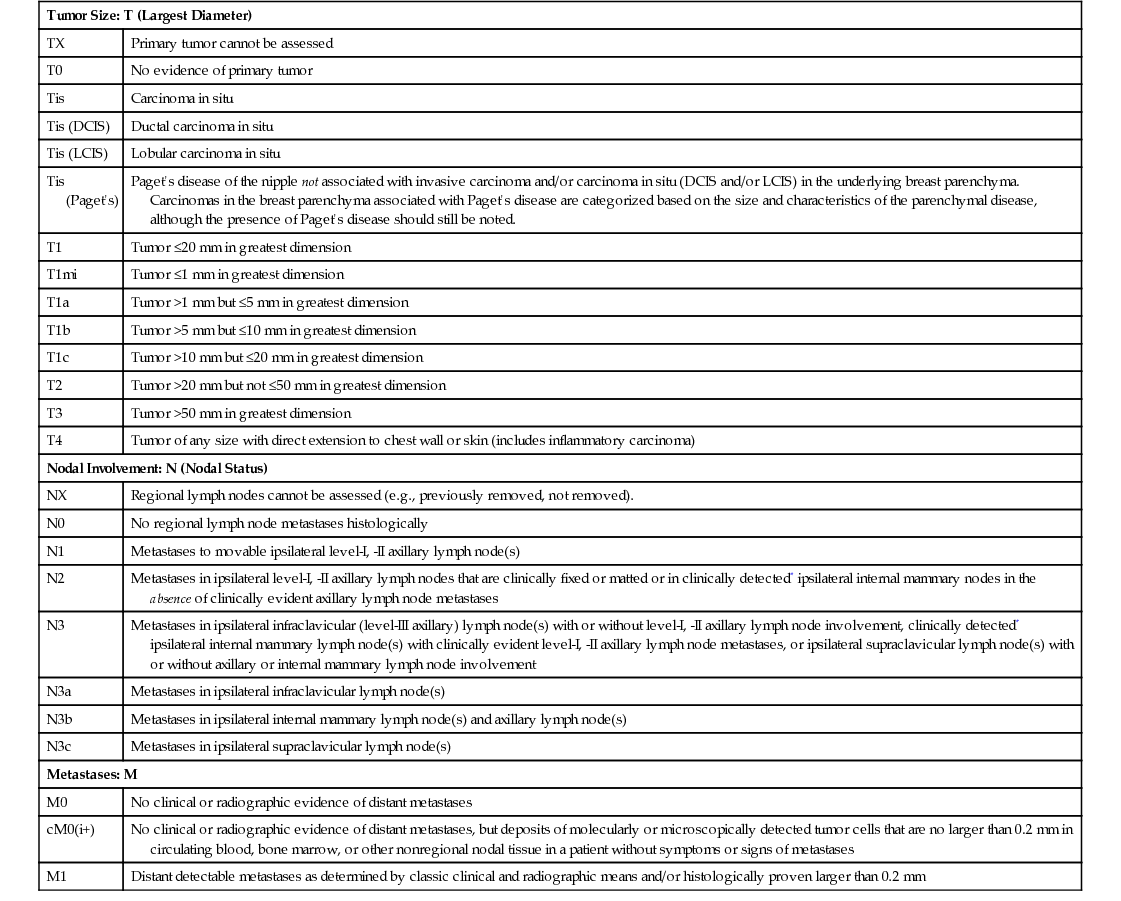
Tumor Size: T (Largest Diameter)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
Tis (DCIS)
Ductal carcinoma in situ
Tis (LCIS)
Lobular carcinoma in situ
Tis (Paget’s)
Paget’s disease of the nipple not associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted.
T1
Tumor ≤20 mm in greatest dimension
T1mi
Tumor ≤1 mm in greatest dimension
T1a
Tumor >1 mm but ≤5 mm in greatest dimension
T1b
Tumor >5 mm but ≤10 mm in greatest dimension
T1c
Tumor >10 mm but ≤20 mm in greatest dimension
T2
Tumor >20 mm but not ≤50 mm in greatest dimension
T3
Tumor >50 mm in greatest dimension
T4
Tumor of any size with direct extension to chest wall or skin (includes inflammatory carcinoma)
Nodal Involvement: N (Nodal Status)
NX
Regional lymph nodes cannot be assessed (e.g., previously removed, not removed).
N0
No regional lymph node metastases histologically
N1
Metastases to movable ipsilateral level-I, -II axillary lymph node(s)
N2
Metastases in ipsilateral level-I, -II axillary lymph nodes that are clinically fixed or matted or in clinically detected* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases
N3
Metastases in ipsilateral infraclavicular (level-III axillary) lymph node(s) with or without level-I, -II axillary lymph node involvement, clinically detected* ipsilateral internal mammary lymph node(s) with clinically evident level-I, -II axillary lymph node metastases, or ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement
N3a
Metastases in ipsilateral infraclavicular lymph node(s)
N3b
Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s)
N3c
Metastases in ipsilateral supraclavicular lymph node(s)
Metastases: M
M0
No clinical or radiographic evidence of distant metastases
cM0(i+)
No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells that are no larger than 0.2 mm in circulating blood, bone marrow, or other nonregional nodal tissue in a patient without symptoms or signs of metastases
M1
Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven larger than 0.2 mm
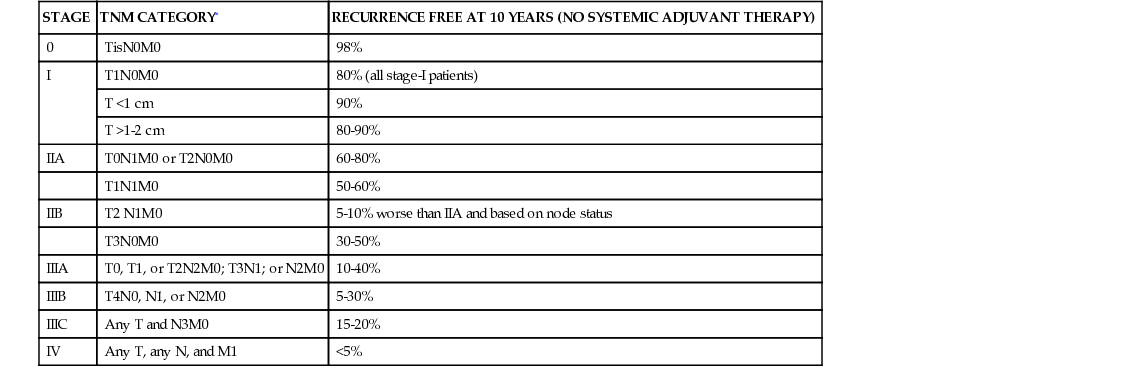
STAGE
TNM CATEGORY*
RECURRENCE FREE AT 10 YEARS (NO SYSTEMIC ADJUVANT THERAPY)
0
TisN0M0
98%
I
T1N0M0
80% (all stage-I patients)
T <1 cm
90%
T >1-2 cm
80-90%
IIA
T0N1M0 or T2N0M0
60-80%
T1N1M0
50-60%
IIB
T2 N1M0
5-10% worse than IIA and based on node status
T3N0M0
30-50%
IIIA
T0, T1, or T2N2M0; T3N1; or N2M0
10-40%
IIIB
T4N0, N1, or N2M0
5-30%
IIIC
Any T and N3M0
15-20%
IV
Any T, any N, and M1
<5%
Breast Cancer and Breast Reconstruction
Chapter 9