Breast Augmentation with Anatomic, High-Cohesiveness Silicone Gel Implants (European Experience)
Per Hedén
The desire of women to have more youthful and shapely breasts can be traced a long way back in human history. The female breast is strongly connected to human sexuality and under appropriate conditions sends a signal of fertility and youth to the opposite sex. It is therefore not surprising that breast augmentation surgery for cosmetic reasons was performed already in the late nineteenth century (1). Since the introduction of silicone gel breast implants in 1964 (2), a steadily increasing demand for these procedures has followed.
Traditionally and throughout the first three decades of silicon implant breast augmentation, the approach to the procedure was volumetric. However, a clear trend toward more dimensional thinking in breast augmentation has evolved since the 1990s. Breast augmentation in the twenty-first century is at a turning point. In the traditional techniques, implant selection was based more on the surgeon’s experience and the patient’s desires than on measurements and tissue analysis. The preoperative planning of the implant’s positioning was also fairly arbitrary, based on the surgeon’s experience. Implant alternatives were limited to round, smooth ones with one or few shapes. In the new era of breast augmentation surgery, implant selection is much more precise, based on a careful analysis of the chest and glandular tissue. Instead of implant selection based on volume, three-dimensional thinking is applied. Implant positioning during surgery is no longer arbitrary and is much more precise, based on careful analysis and measurement. Many different implants are also available. Besides the traditional round ones, anatomic and asymmetric implants are available. Implants can have a smooth or textured surface and are available in several different shapes with varying heights, widths, and projections.
Current filling material in breast implants, however, has changed little since the 1960s. In the United States saline is still the dominant filler due to the silicone moratorium imposed by the Food and Drug Administration (FDA) in 1992. Silicone-filled implants have recently been reintroduced in the United States, and a rapid growth in their use has been noted. Silicone gel dominates as implant filling material in the rest of the world. During the 1990s the characteristics of silicon fillings changed, however, resulting in implants with higher cohesiveness. This has resulted in greater shape-retaining properties. Thus the fundamental difference between traditional silicone gel–filled implants and these new high-cohesiveness silicone implants is their form stability. Recently round, high-cohesiveness silicone gel implants have also been introduced.
This chapter discusses how to select form-stable implants and make preoperative markings, as well as how much the implant shape and filling material matter. It is important to realize that form-stable implants behave considerably differently than non–form-stable implants. Surgeons who use them in similar ways are bound to have more complications and increase the risk for reoperations. Indeed, not only are the implant selection and surgical technique different, the patient communication, the preoperative markings, and the postoperative recommendations also differ. Form-stable implants provide much better chances to control the shape of the new breast, and we in the twenty-first century have entered into a new breast-shaping era as opposed to the old breast-stuffing era.
Anatomic, High-Cohesiveness Silicone Gel Breast Implants: Manufacturers
In 1994 the McGhan Corporation (later Inamed and now a part of Allergan) was the first manufacturer to introduce anatomic, high-cohesiveness silicon breast implants (AHCSI). The brand name was Style 410. This was followed by the introduction of a family of different shapes of these implants (the Matrix system). The first eight patients in the world were operated with this Matrix system at Akademikliniken in Stockholm in 1997, and since then our group has gained leading international experience with these implants. In 2008 more than 10,000 of these had been implanted, and several international postgraduate courses, workshops, and demonstration operations had been held. The Allergan Matrix Style 410 implants are filled with a highly cohesive silicone gel, which results in the stability of their form. The implant shapes are tear dropped, anatomic, and available in 12 different sets of heights and projections (a given combination of height and projection is called a cell). Height varies from low to moderate to full, and projection from low to moderate to full to extra projecting. Thus, a moderate-height implant with a low projection is called an ML implant, and a full-height implant with extra projection is called an FX model (Fig. 115.1). These implants are soft but slightly firmer than regular low-cohesiveness (responsive) silicone gel implants. When these AHCSI implants are cut in half, the material is slightly sticky on direct contact but shape retaining. In contrast to a standard silicone gel breast implant, the material will not leave the shell during manual compression of a cut implant. Much of the discussion in this chapter is based on experience in the use of Allergan Style 410 implants but is also applicable to other types of implants. Since McGhan/Allergan introduced AHCSIs, other manufacturers (e.g., Mentor, Eurosilicone, Polytech/Silimed, Perthese, and Arion) have also introduced AHCSIs. The Matrix system with 12 different
implant cells is exclusive to Allergan, but other manufacturers have systems of form-stable anatomic implants with varying heights and projection. Mentor, which recently became a part of Johnson & Johnson, has an anatomic, high-cohesiveness silicone gel implant style called CPG (Contour Profile) available in five different shapes with varying heights and projection. Allergan has also introduced a new type of form-stable anatomic implant called Style 510. This implant contains two different cohesive silicone gels. The ventral part is considerably firmer than the posterior part, with the aim to maintain extra projection in the nipple-areola complex. AHCSIs of different manufacturers have different properties of the silicone gel, and the cohesiveness characteristics may vary from one type of implant to another. No standardized nomenclature describing the degree of cohesiveness has been developed by the industry, and thus it is difficult to make comparisons among manufacturers. A degree of confusion exists regarding “cohesive” silicone gel implants because all silicone implants by definition are cohesive. The liquid state only exists where molecules are relatively free from each other as in water or saline solution. Thus even a silicone gel implant that has “flowing” properties is cohesive. However, it is low in cohesiveness. A simple way to get an idea of the degree of cohesiveness of a silicone breast implant is to do a so-called tilt test: The implant is placed flat in the horizontal hand of the examiner and then turned vertical by tilting the tip of the implant with the fingers; the degree of shape-retaining properties is then noted. The lower the cohesiveness, the more one will note collapses of the upper pole of the implant.
implant cells is exclusive to Allergan, but other manufacturers have systems of form-stable anatomic implants with varying heights and projection. Mentor, which recently became a part of Johnson & Johnson, has an anatomic, high-cohesiveness silicone gel implant style called CPG (Contour Profile) available in five different shapes with varying heights and projection. Allergan has also introduced a new type of form-stable anatomic implant called Style 510. This implant contains two different cohesive silicone gels. The ventral part is considerably firmer than the posterior part, with the aim to maintain extra projection in the nipple-areola complex. AHCSIs of different manufacturers have different properties of the silicone gel, and the cohesiveness characteristics may vary from one type of implant to another. No standardized nomenclature describing the degree of cohesiveness has been developed by the industry, and thus it is difficult to make comparisons among manufacturers. A degree of confusion exists regarding “cohesive” silicone gel implants because all silicone implants by definition are cohesive. The liquid state only exists where molecules are relatively free from each other as in water or saline solution. Thus even a silicone gel implant that has “flowing” properties is cohesive. However, it is low in cohesiveness. A simple way to get an idea of the degree of cohesiveness of a silicone breast implant is to do a so-called tilt test: The implant is placed flat in the horizontal hand of the examiner and then turned vertical by tilting the tip of the implant with the fingers; the degree of shape-retaining properties is then noted. The lower the cohesiveness, the more one will note collapses of the upper pole of the implant.
To minimize the risk for rotation of anatomic implants, the surface of the implant shell is textured. The purpose of this texturing is to allow tissue in-growth, immobilization, and a reduced frequency of capsular contraction. Indeed, surface texturing may permit tissue in-growth and adherence to the surrounding tissue, thereby facilitating implant immobility (3,4,5). Texturing also prevents circumferential linear fibrosis associated with capsular contraction (6). This effect is only clear in studies of implants in the subglandular position. Different manufacturers of AHCSIs have different techniques to produce the texturing of their implants. To aid tissue in-growth and adherence, a pore diameter of greater than 300 μm is regarded as a prerequisite (7). The BioCell surface of Style 410 implants has a pore size between 250 and 750 μm and a mean pore diameter of 500 μm. The Mentor implant has a pore size of less than 300 μm in its textured Siltex surface, which does not permit tissue in-growth. However, there is no indication that the pore size has any effect on the degree of capsular contracture.
Indications for High-Cohesiveness Silicone Gel Breast Implants
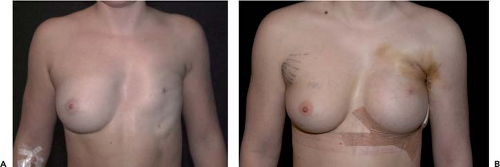
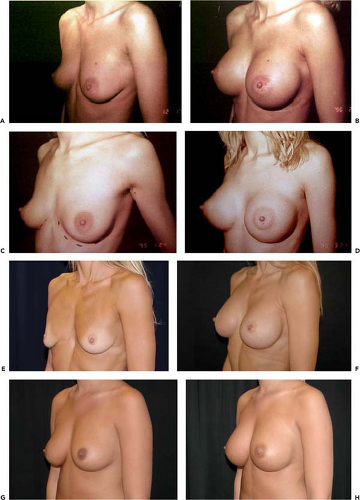
The main indications for AHCSIs are primary breast augmentations in women who desire a proportionate breast augmentation. This augmentation may be of greater, lesser, or moderate extent but still proportionate to the dimensions of the chest and glandular tissue. AHCSIs are not suitable for women who desire a very large and nonproportionate breast augmentation with an artificial appearance. AHCSIs are also useful in secondary breast augmentation but may be more technically demanding than standard low-cohesiveness breast implants or liquid-filled implants. In a secondary breast augmentation it is recommended that a new implant pocket be created to minimize the risk for rotation of the implant. If severe capsular contraction is present, it is possible that the atrophy of the glandular tissue is pronounced. If this is the case, it may be difficult to fill the dead space after the old implant with a high-cohesiveness filler. The use of implants without shape-retaining properties may be advantageous in these cases. An alternative may be to use a combined mastopexy augmentation and an AHCSI. A third clear indication for AHCSI is breast reconstructive surgery. The wide variety of implant shapes provides a greater degree of individualization and variation than other implants on the market. This is of extra value when correcting breast asymmetries. Congenital deformations such as Poland syndrome (Fig. 115.2) and postmastectomy defects are examples of very pronounced asymmetries that can benefit from the great range of choices of different implant shapes. The stable mound of a high-cohesiveness silicon implant also permits better skin redraping and contracture after a subcutaneous mastectomy than a low-cohesiveness implant or liquid filler, which would tend to flow out into the lower part of the dissected pocket (Figs. 115.3 and 115.4).
Advantages and Disadvantages of High-Cohesiveness Silicone Gel Breast Implants
The large number of form-stable implant shapes available makes a more customized breast augmentation possible compared to the traditional breast augmentation technique. This is obviously better, as a great variation exists in patient desires, thoracic shapes, and amount of glandular tissue. An obvious advantage of this variation in shapes exists in the treatment of breast asymmetries. By careful analysis of the thoracic and glandular differences between right and left sides, the prerequisites for different implant shapes can be established. A computed tomography scan can be useful in evaluating thoracic asymmetries for detailed analysis of the chest wall shape (Fig. 115.5). A newer tool in the analysis of breast shapes is three-dimensional imaging.
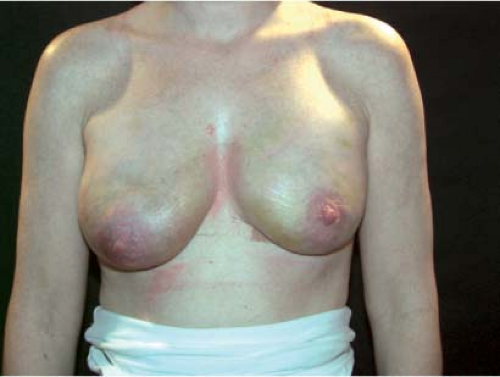 Figure 115.4. Same case as in Figure 115.3 at 1 week after surgery. Note the nipple position and compare it to the situation 6 months later (Fig. 115.3B). |
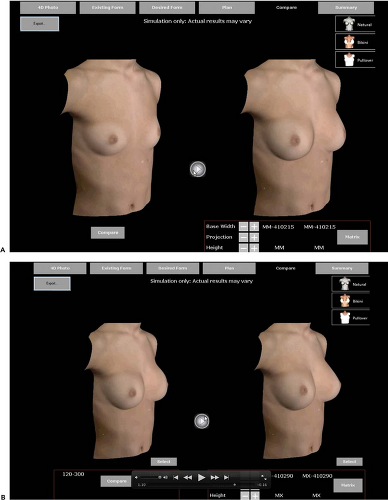
Today this permits analysis of existing breast shape, and systems for prediction of the outcome of a breast augmentation using differently shaped implants are under development. This could be an extremely valuable tool for the planning of a breast augmentation, as well as for patient communication in advance of surgery (Fig. 115.6).
The FDA moratorium on silicone gel implants in 1992 raised three main concerns:
Risk for breast cancer
Risk for autoimmune disease
Implant rupture
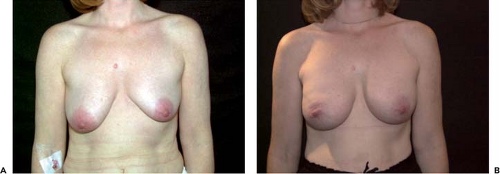
Since 1992 it has clearly been documented that breast augmentation does not increase the risk for breast cancer (8,9,10,11). It has also been well documented in large epidemiologic studies that no increased risk for autoimmune disease exists after implantation of silicone gel implants (12,13,14,15). However, the true frequency of implant rupture is unknown and varies greatly with the generation of implants. One advantage with AHCSIs is that there is less movement of the implant shell due to the nature of the filling material. Implants with less movement of the shell are likely to wear less and may have a longer life expectancy. We have demonstrated a very low rupture frequency of high-cohesiveness silicone gel implants with implantation time of up to 9 years. Comparison of the results from this magnetic resonance imaging study with the rupture frequency of low-cohesiveness silicone-filled implants with similar implantation time shows that low-cohesiveness fillers may have between 5 and 30 times higher rupture risk (16). If a shell rupture occurs in an implant with a high-cohesiveness filler, it is even possible that this may not produce negative effects because the form-stable filling material is less likely to leak out through small holes in the shell. Another advantage with AHCSIs is that more force is needed to deform the implants compared to a low-cohesiveness or liquid filler. If capsular contraction occurs, this is likely to result in less deformation, and this has also been our clinical experience (Fig. 115.7). Implants with low-cohesiveness fillers are easily deformed into a spherical shape when compressed (Fig. 115.8).
High-cohesiveness implants also have less tendency to wrinkle even if surface irregularities can been seen. The upper pole of an AHCSI is the area that is most prone to irregularities because this is the thinnest part of the device. Due to their shape, full-height implants with low projection have the weakest upper pole, and these are the models with the highest risk for upper pole irregularities. The risk increases if the upper pole of the implant pocket is underdissected and implants are placed in the subglandular position in thin patients. A more pronounced irregularity with buckling of the upper pole has been reported (17) and may be attributed to the aforementioned factors.
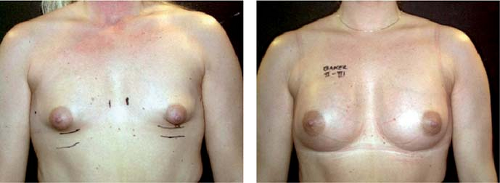
If similar-shaped breasts with similar amounts of glandular tissue are treated with implants of different shapes and the implants are placed in the same implant position in relation to the pectoralis muscle and are found to be without capsular contraction on late follow-up, it has been noted that anatomic implants produce a more natural upper pole than round ones (Fig. 115.9). It has been claimed that round implants change into anatomic shape in the standing position (18). This is only true if the device is underfilled and filled with a low-cohesiveness or liquid filler and if no capsular contraction is present. However, this also results in collapse of the upper pole with irregularities of the shell. This situation is also likely to result in more envelope wear and probably long-term increased rupture risk. If a capsular contraction develops, these implants will deform into a spherical and unnatural shape. If the devices are overfilled to reduce these problems, they become more rounded and unnatural in shape. They will also be firmer. Thus the implant shape and filling material clearly make a difference in the final outcome of a breast augmentation procedure.
What are the indications for round breast implants? The primary indication is the patient’s preference, especially in women who desire a more rounded and artificial appearance. It may also be an economic consideration for the patient because most AHCSI implants are considerably more expensive than round ones. For patients who desire the softest implant available, a low-cohesiveness, round silicone implant may also be offered. The patient should be informed, however, that a common small degree of capsular contraction activity is much
more important for the firmness of the breast than the consistency of the implants. It should also be noted that AHCSIs are available with different degrees of firmness (e.g., the Allergan Style 410 Soft Touch implant). Another indication for round implants is recurrent rotation of anatomic implants. Round implants may also be recommended in selected secondary cases, especially, as mentioned earlier, in severe unilateral capsular contraction cases. In these cases low-cohesiveness filler or a combination of cohesiveness filler with mastopexy may be recommended. Recently, round, form-stable implants (e.g., Allergan Inspira TRM, TRF, and TRX) have been introduced. These behave similar to anatomic form-stable, high-cohesiveness implants, but due to their shape obviously do not have the risk for rotational problems. They may also be favored in situations in which patients want a fuller upper pole.
more important for the firmness of the breast than the consistency of the implants. It should also be noted that AHCSIs are available with different degrees of firmness (e.g., the Allergan Style 410 Soft Touch implant). Another indication for round implants is recurrent rotation of anatomic implants. Round implants may also be recommended in selected secondary cases, especially, as mentioned earlier, in severe unilateral capsular contraction cases. In these cases low-cohesiveness filler or a combination of cohesiveness filler with mastopexy may be recommended. Recently, round, form-stable implants (e.g., Allergan Inspira TRM, TRF, and TRX) have been introduced. These behave similar to anatomic form-stable, high-cohesiveness implants, but due to their shape obviously do not have the risk for rotational problems. They may also be favored in situations in which patients want a fuller upper pole.
 Figure 115.8. A: Implants with low-cohesiveness fillers are easily deformed into a spherical shape when compressed. Baker III contraction. On the right the implant is seen with its intact capsule. The capsule on the left implant has been removed. The implants (Inamed Style 110) are of equal shape size and type. B: Low-cohesiveness silicone gel implants (Style 110) and a similar degree of firmness as the right breast in Figure 115.7 but considerably more deformed. |
Other disadvantages of AHCSIs include higher costs and more technically demanding procedures with a steeper learning curve. Incisions also need to be a slightly longer (4 to 6.5 cm, averaging 5.5 cm, depending on implant dimensions and size) because of the characteristics of the filling material. High-cohesiveness implants should not be forced through a too-small hole. AHCSIs are also firmer in Baker I cases and not suitable for exaggerated and nonproportionate augmentations. Finally, a risk for rotation exists with these implants. However, the frequency has been reported to be as low as 0.42% (19). It also has to be recognized that nonadhesion of tissue on the implant surface does not always result in rotation. A textured implant surface with a pore size larger than 300 μm is a prerequisite for in-growth of tissue and fixation of the implant. Some anatomic form-stable implant surfaces (e.g., the Siltex of Mentor/Johnson & Johnson implants) have a pore size smaller than 300 μm and thus do not permit tissue fixation (7). However, despite this, it is rotation is also relatively uncommon with these anatomic high-cohesiveness, form-stable implants.
Most rotations were seen in secondary cases, and to avoid these problems anatomic implants should not be implanted into old implant pockets in secondary augmentation surgery. This is especially true if the implant pocket has been formed around a smooth-walled implant. It is believed that the smooth-walled pocket does not permit tissue in-growth into the texturing of the implant and thus rotation is facilitated. Rotation may also be seen in primary augmentation surgery. In these cases the implants are not immobilized, and this may have several possible explanations. The reasons for poor tissue immobilization of the implants are not clearly known, but several possible explanations can be offered. The problem may be related to poor surgical technique with dissection of a too-large implant pocket. To minimize this risk for an overdissected implant
pocket, it could be checked with a ruler during the procedure. The goal is a snug-fitting implant pocket. The width of the pocket only marginally should exceed the implant width. Less than 1 cm of dissection on each side is recommended. However, the height of the implant pocket should exceed the implant height by at least 2 cm or slightly more to allow good redraping of the tissues in the upper pole of the implant. Other possible explanations for late rotations of anatomic implants may be the formation of a so-called pseudocapsule or double capsule (Fig. 115.10). This is clinically noted as a complete separation of the capsule into two layers. The inner one is complete or partial and sticks on the walls of the textured implant. A small amount of serous material may be noted between the two layers of the capsule. No explanation of these phenomena is available in the literature, but several possible hypotheses may be offered. One is that a subclinical infection with the formation of a biological film on the implants is present. In support of this theory is the observation that only one side may be affected and that the situation can be effectively treated with the creation of a new implant pocket and exchange of the implant. Another possible explanation is that seroma is formed around the implant early after implantation, and contributing to this could be a poor surgical technique with creation of too large an implant pocket or traumatic blunt dissection. The dissection should be sharp, bloodless, and without overdissection. The use of tungsten carbide needle cautery (Colorado tip) is recommended. In the setting of submuscular implantation, the loose connective tissue between ribs and muscle should be left on the ribs to minimize seroma formation. Another possible explanation for rotation of anatomic implants is trauma or excessive motion. Support for this theory comes from noting that this has been mostly observed in patients with loose parenchyma and an envelope where the implant has more opportunity for movement. It has also been noted in secondary cases that the smooth patch on the back side of Style 410 implants often is surrounded by a slightly larger area of nonadhesion. It could be argued that this is a weak point of the implants and could be the starting point for seroma formation and dissection of the implant capsule into two separate layers.
pocket, it could be checked with a ruler during the procedure. The goal is a snug-fitting implant pocket. The width of the pocket only marginally should exceed the implant width. Less than 1 cm of dissection on each side is recommended. However, the height of the implant pocket should exceed the implant height by at least 2 cm or slightly more to allow good redraping of the tissues in the upper pole of the implant. Other possible explanations for late rotations of anatomic implants may be the formation of a so-called pseudocapsule or double capsule (Fig. 115.10). This is clinically noted as a complete separation of the capsule into two layers. The inner one is complete or partial and sticks on the walls of the textured implant. A small amount of serous material may be noted between the two layers of the capsule. No explanation of these phenomena is available in the literature, but several possible hypotheses may be offered. One is that a subclinical infection with the formation of a biological film on the implants is present. In support of this theory is the observation that only one side may be affected and that the situation can be effectively treated with the creation of a new implant pocket and exchange of the implant. Another possible explanation is that seroma is formed around the implant early after implantation, and contributing to this could be a poor surgical technique with creation of too large an implant pocket or traumatic blunt dissection. The dissection should be sharp, bloodless, and without overdissection. The use of tungsten carbide needle cautery (Colorado tip) is recommended. In the setting of submuscular implantation, the loose connective tissue between ribs and muscle should be left on the ribs to minimize seroma formation. Another possible explanation for rotation of anatomic implants is trauma or excessive motion. Support for this theory comes from noting that this has been mostly observed in patients with loose parenchyma and an envelope where the implant has more opportunity for movement. It has also been noted in secondary cases that the smooth patch on the back side of Style 410 implants often is surrounded by a slightly larger area of nonadhesion. It could be argued that this is a weak point of the implants and could be the starting point for seroma formation and dissection of the implant capsule into two separate layers.
Common arguments against the use of anatomic implants include that these are too expensive and that patients are happy with traditional round, less cohesive gel-filled implants. It has also been stated that high-cohesiveness fillers are too firm and do not move naturally on the chest wall. AHCSIs are indeed more expensive. However, as these implants appear to have a longer life span, they may actually be cheaper per time unit. Dividing the extra cost of AHCSIs by the number of years that such implants are likely to be in place results in a fairly small annual cost. This argument is easily accepted by patients, and most prefer an extra cost for something they “wear” every day. It is also true that most patients are happy with traditional round silicone gel implants, but it should been remembered that many patients are happy with inferior results and that this is no reason plastic surgeons should refrain from improvements. In fact it is their obligation as surgeons to try to do their best. Patients should be happier with an even better result.
Careful analysis of the consistency of an augmented breast reveals that a low grade of capsular activity is more common than usually noted by the surgeon or patient. If a breast judged to be without capsular contraction is compressed and this is compared to the implant material external to the body, it is commonly noted that the breast is slightly firmer. This much more commonly results in firmness of the breast than the actual firmness of the implant. It has also been noted that the grade of capsular activity is a much more important factor for the movement of the breast on the chest wall than the firmness of the implant itself (Fig. 115.11). The AHCSI without clear capsular contracture moves as freely on the chest wall as any other implant type. I conclude this section on the advantages and disadvantages of the AHCSI by stating that in my 30 years of experience in breast augmentation surgery, this type of implant has given higher patient satisfaction and a lower frequency of complications than all other implant types and techniques I have used.
Implant Selection: Preoperative Analysis and Measurements
In spite of the fact that dimensional thinking was introduced in breast implant surgery during the early 1990s (19,20), it is still very difficult to get patients, nurses, and plastic surgeons to
stop thinking of implant volume as the most important factor in implant selection. Many surgeons still arbitrarily select and suggest an implant volume based on feelings and experience rather than on measuring and analyzing the breast prior to implant selection. Today, however, breast augmentations are much more about proportions and dimensions than implant volume. This can be illustrated by comparing patients with a large-looking breast but a small implant volume and patients with a moderately sized breast but a larger implant volume (Fig. 115.12). Patients should be led to understand that the final appearance of the augmented breast is highly related to the initial amount of breast tissue, its dimensions, and the size of the chest wall and that they should not select an implant based on a preconceived idea about a suitable volume. Many
patients have reviewed the Internet before a consultation, and therefore it is not uncommon that they have a faulty idea about the desired implant volume. It is fundamentally important that patients understand that implants have to suit the biological conditions and that implant selection has to be tissue based.
stop thinking of implant volume as the most important factor in implant selection. Many surgeons still arbitrarily select and suggest an implant volume based on feelings and experience rather than on measuring and analyzing the breast prior to implant selection. Today, however, breast augmentations are much more about proportions and dimensions than implant volume. This can be illustrated by comparing patients with a large-looking breast but a small implant volume and patients with a moderately sized breast but a larger implant volume (Fig. 115.12). Patients should be led to understand that the final appearance of the augmented breast is highly related to the initial amount of breast tissue, its dimensions, and the size of the chest wall and that they should not select an implant based on a preconceived idea about a suitable volume. Many
patients have reviewed the Internet before a consultation, and therefore it is not uncommon that they have a faulty idea about the desired implant volume. It is fundamentally important that patients understand that implants have to suit the biological conditions and that implant selection has to be tissue based.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree