Brava and Autologous Fat Transfer Is a Safe and Effective Breast Augmentation Alternative: Results of a 6-Year, 81-Patient, Prospective Multicenter Study
Roger K. Khouri, M.D.
Marita Eisenmann-Klein, M.D.
Eufemiano Cardoso, M.D.
Brian C. Cooley, Ph.D.
Daniel Kacher, M.S.
Eva Gombos, M.D.
Thomas J. Baker, M.D.
Key Biscayne and Miami, Fla.; Regensburg, Germany; Milwaukee, Wis.; and Boston, Mass.
From the Division of Plastic Surgery, Florida International University; the Miami Breast Center; Klinik für Plastische und Ästhetische Hand- und Wiederherstellungschirurgie, Caritas-Krankenhaus St. Josef; Orthopaedic Surgery, Medical College of Wisconsin; Surgical Planning Laboratory and Radiology Breast Imaging, Brigham and Women’s Hospital, Harvard Medical School; and the Department of Surgery, University of Miami.
Received for publication August 23, 2011; accepted November 29, 2011.
Preliminary study results presented at the Annual Congress of the American Society for Aesthetic Plastic Surgery, in Orlando, Florida, May 21 through 25, 2006; interim results presented at the Annual Congress of the American Society of Plastic Surgeons, in Seattle, Washington, October 23 through 27, 2009.
Copyright ©2012 by the American Society of Plastic Surgeons
DOI: 10.1097/PRS.0b013e31824a2db6
Disclosure: Dr. Khouri has an equity interest in Brava, LLC, the manufacturer of the Brava device, and is an owner of the company that makes the Lipografter described in the article. The other authors have no financial interests to disclose.
Background: Breast augmentation by autologo us fat transfer is an appealing alternative in need of scientific validation.
Methods: In a prospective multicenter study, 81 women (age range, 17 to 63 years) wore the Brava device, a bra-like vacuum-based external tissue expander, for 4 weeks and then underwent autologous fat injection using 10 to 14 needle puncture sites into each breast in a three-dimensional fanning pattern (average, 277 ml volume injected per breast). Patients resumed Brava wear within 24 hours for 7 or more days. Pretreatment and posttreatment breast volumes were derived from three-dimensional volumetric reconstruction of magnetic resonance imaging scans, and outcomes were compared with a meta-analysis of six recent published reports on autologous fat transfer breast augmentation without expansion. Follow-up ranged from 12 months to 6 years (average, 3.7 years).
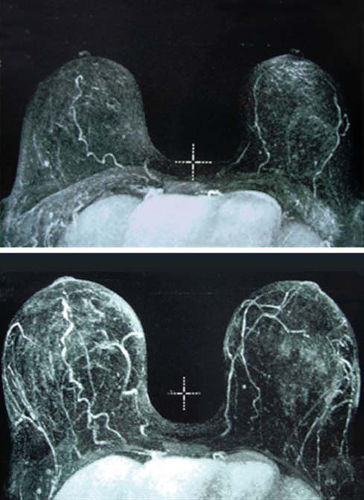
Results: Breast volume was unchanged between 3 and 6 months. Seventy-one of the treated women were compliant with Brava wear and had a mean augmentation volume at 12 months of 233 ml per breast compared with 134 ml per breast in published series without Brava (p < 0.00001). Graft survival was 82 ± 18 percent compared with 55 ± 18 percent without Brava (p < 0.00001). There was a strong linear correlation (R2 = 0.87) between pregrafting Brava expansion and the resultant breast augmentation. There were no suspicious breast masses or nodules. Magnetic resonance imaging recognized a 16 percent incidence of fat necrosis easily identified at 1-year mammographic evaluation.
Conclusion: The addition of Brava expansion before autologous fat grafting leads to significantly larger breast augmentations, with more fat graft placement, higher graft survival rates, and minimal graft necrosis or complications, demonstrating high safety and efficacy for the procedure. (Plast. Reconstr. Surg. 129: 1173, 2012.)
CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, IV.
Autologous fat transfer to the breast has a long and controversial history.1,2 In 1987, a position statement by the American Society of Plastic Surgeons3 banned the procedure out of concern that the grafts would not survive and could lead to calcification believed to be indistinguishable from cancer with the xeromammographic technology of the time. However, radiologists today are better able to differentiate neoplastic processes from fat necrosis.4–6 Furthermore, because of many technical refinements,7,8 autologous fat transfer today holds much promise in plastic surgery.9–24 Therefore, in 2007, the American Society of Plastic Surgeons commissioned a Fat Graft Task Force that concluded that autologous fat transfer might be used for the breast “while the techniques and the results vary…. leaving a tremendous need for high quality clinical studies.”25 In 2009, the American Society of Plastic Surgeons lifted the ban on fat grafting for breast reconstruction while recommending cautious use for augmentation26 because of concern for safety and efficacy, given the paucity of scientific studies.
Breast augmentation with liposuctioned fat has suffered from two fundamental limitations: the volume of fat that can be transferred in a single session and the percentage graft survival.18–22,27 In fact, there seems to be an inverse relationship between the two (i.e., the more fat grafted, the lower its survival rate).28 Efforts at overcoming this have focused on harvesting techniques, fat manipulation, stem cells, and related approaches.13,17–20,23,24,27,29–72 Most studies report 50 to 60 percent survival and an augmentation in the 100-ml range on long-term follow-up.17–22,27 Of note, none made any attempt to improve the quality of the recipient breast.
To preserve the graft-to-recipient interface critical for revascularization and survival, fat grafts have to be dispersed as microdroplets. Because in the small breasts to be augmented there is physically no room for dispersal without crowding a large quantity of microdroplets, we postulated that preparation of the recipient breast by external expansion is the key missing ingredient.
The Brava device has been on the market for over 10 years as an external soft-tissue expander and has demonstrated modest, permanent augmentation after long-term use.73–77 Short-term use of Brava, however, causes a marked temporary increase in breast size and generates a very large fibrovascular scaffold that would be an ideal recipient for fat grafts (Khouri RK, personal observation). We undertook this multicenter, prospective, magnetic resonance imaging–documented study to determine the safety and efficacy of single-stage large-volume autologous fat transfer to the breast treated with the Brava external breast expander.
Patients and Methods
This study was designed to optimize all potential variables. This includes low-pressure atraumatic fat harvest, minimal graft manipulation, and meticulous microdroplet grafting. Because a larger recipient has room in which to safely graft larger volumes and because it is well proven that Brava expansion enlarges the recipient breast, we found it unethical to randomize Brava patients versus nonexpanded controls and arbitrarily condemn women to the morbidity and risks of surgery for a less effective procedure. Furthermore, because there are multiple recent peer-reviewed reports of autologous fat transfer breast augmentation without expansion, we elected to compare our Brava-expanded cohort to a meta-analysis of this well-established baseline.
On institutional review board approval (Concordia Clinical Research, Inc.; Breast Reconstruction and Augmentation with Brava Enhanced Autologous Fat Micro Grafting Protocol No. 2004-2, IRB COMM. No. 167), 81 women (Miami Breast Center, Key Biscayne, Fla., n = 59; Caritas-Krankenhaus St. Josef, Regensburg, Germany, n = 12; Harley Medical Center, London, United Kingdom, n = 10) who desired breast augmentation, were averse to implants, and who tolerated a 20-minute Brava test trial in the office were enrolled in the study. We performed 77 bilateral and four unilateral autologous fat transfer breast augmentations on 170 breasts. Patient ages ranged from 17 to 63 years and body mass index ranged from 15 to 28 (average, 19.8). Smokers were excluded. All enrolled were grafted despite wide variation in compliance with the requested pregraft Brava treatment1 and despite the fact that four patients were noncompliant. Six patients did not return for follow-up magnetic resonance imaging, and although self-reports indicate they are complication-free, postprocedure breast volumetric measurements were not taken. Six of the earlier patients later underwent grafting a second time. However, we only analyzed the outcome of their first graft. Figure 1 shows the breakdown of the treated and compliant patient groups.
Before Brava expansion and in phase with her menstrual cycle, every woman underwent baseline magnetic resonance imaging with breast coils, intravenous gadolinium contrast, and fat subtraction. The patients were asked to wear the Brava external breast tissue expander for 10 hours/day for 4 weeks. This preexpansion period increases the vascularity of the recipient site.61,62,78 For the last 36 to 48 hours, they were asked to maintain uninterrupted expansion and come to the operating room still wearing the expander, to induce an immediate temporary three-dimensional enhanced enlargement of the subcutaneous periglandular tissue matrix (Fig. 2).
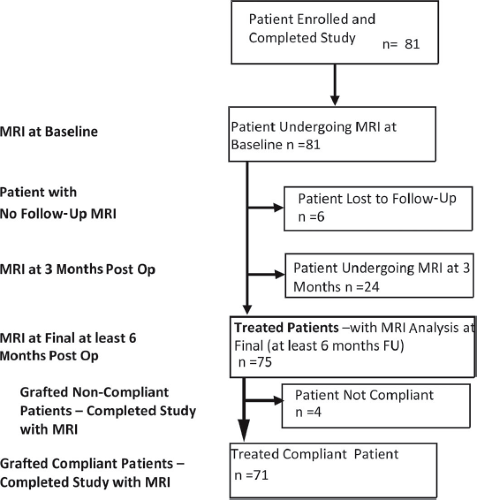 Fig. 1. Study design flowchart, showing sequence of magnetic resonance imaging (MRI) scans, with breakdown of numbers based on follow-up (FU) and Brava use compliance. |
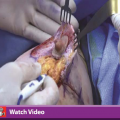
Harvesting and grafting were performed with the Lipografter, a closed fat harvesting, processing, and grafting device (KVAC Syringe and A-T Valve; Lipocosm, LLC, Miami, Fla.). The fat was aspirated with a 12-hole, 2.7-mm cannula (Marina Medical, Sunrise, Fla.) attached to a spring-activated KVAC syringe pulling a constant 300-mmHg vacuum. The aspirate was transferred directly from the syringe to a collection bag through a nonclogging three-way A-T Valve and the bags were centrifuged at 15 g for 3 minutes. The supernatant fat was then reinjected directly from the bag using the A-T Valve in reverse using 3- to 5-ml syringes and 2.4-mm single-sidehole blunt 15- to 25-cm reinjection cannulas. We grafted the breast through a multitude of perimammary and periareolar needle puncture sites, injecting no more than 1 ml per 5 cm of cannula retraction, microweaving the grafts and fanning the passes radially around each injection site. Adequate preexpansion allowed us to layer the grafts in three planes, the immediate subdermal, the deeper mastectomy level, and an intermediate subcutaneous plane. We avoided the peau d’orange effect of subcutaneous overfilling. We then proceeded to graft the subglandular tissue, the pectoral muscle, and the subpectoral plane, strictly avoiding the breast parenchyma. We carefully avoided localized collections and overgrafting as assessed by tissue turgor. A supportive conforming breast bandage was applied at the end of the procedure.
Within 24 hours after the procedure, patients removed all dressings, took a shower, and wore the Brava device for the next 48 to 72 hours uninterrupted to hold up the grafts as stents during the revascularization and early engraftment period. On the third postoperative day, they were encouraged to return to their normal lifestyle and to wear the Brava device only at night for 4 more days. If Brava use was well-tolerated, they continued wearing it a few hours per day, tapering the wear over an additional few weeks. Patients were seen on a quarterly basis for the first year and then only on an as-needed basis. Final follow-up was by means of electronic mail or telephone. At 3 months after grafting, a second magnetic resonance imaging scan was obtained on the first 24 patients, and all underwent final magnetic resonance imaging at 6 to 12 months. All women older than 40 years underwent mammography at 1 year complemented by an ultrasound examination whenever indicated by the radiologist. Two independent teams of breast radiologists reviewed the mammograms and magnetic resonance imaging scans.
Baseline and final breast volume measurements were derived from magnetic resonance imaging scans viewed in axial orientation with the Digital Imaging and Communications in Medicine standard. The breast area was
outlined for sections at 1-mm intervals, including the skin and basing the internal margin on consistent anatomical landmarks (e.g., sternum, pectoralis, shoulder features). Areas were summed to yield a volume approximation for each breast, measured in milliliters.79 Maximal expansion volume was derived photographically by comparing the standard set of three poses obtained at the time of maximal expansion on the day of surgery with two other sets of the exact same three poses taken at the baseline and at the final breast volume measurements, both with known magnetic resonance imaging–derived measurements. The injected graft volumes were recorded during the procedure.
outlined for sections at 1-mm intervals, including the skin and basing the internal margin on consistent anatomical landmarks (e.g., sternum, pectoralis, shoulder features). Areas were summed to yield a volume approximation for each breast, measured in milliliters.79 Maximal expansion volume was derived photographically by comparing the standard set of three poses obtained at the time of maximal expansion on the day of surgery with two other sets of the exact same three poses taken at the baseline and at the final breast volume measurements, both with known magnetic resonance imaging–derived measurements. The injected graft volumes were recorded during the procedure.
Statistical analysis was performed on three endpoints: augmentation volume, defined as final–baseline breast volume measurement; percentage augmentation, defined as [augmentation volume/baseline] × 100; and graft survival rate, defined as [augmentation volume/injected graft volume] × 100. Data extracted from six recently published clinical studies,18–23 which did not use expansion before autologous fat transfer, were combined and used as a control group (total sample size, n = 335).80–82 Of these, four (n = 280) reported autologous fat transfer augmentation using various means of harvesting and fat separation,18,20,21,23 and two (n = 55) used stem cell–enhanced technology (which involves the addition of processed fat and concentrated stem cells).19,22 Table 1 shows the graft retention rates based on outcomes from these studies, with a mean graft retention rate of 55 percent. The data for our series were compared using paired t tests (before treatment versus after treatment). For comparison of the percentage augmentation with the previously published pooled control group, we used a two-sample independent-variance t test.
In addition to the comparison of the mean retention rate and augmentation volumes of the published autologous fat transfer control and our autologous fat transfer plus Brava–treated groups, a dose-response curve was developed to measure the effect of preexpansion on fat volume transferred, using a paired t test. All enrolled women were asked to use the Brava device for 10 hours/day for 4 weeks. However, some were more compliant than others; and some, with involutional atrophy, had tissues that were more compliant than the younger, tighter nulliparous breasts. Thus, we observed a marked variability in the amount of pregraft breast expansion that allowed us to build a dose-response curve of expansion versus augmentation.
To further analyze the relationship between expansion and augmentation, a regression analysis was performed on the sample of 75 women. The data were normalized by dividing both variables by baseline volume. Maximal expansion/baseline volume was used as the independent variable and augmentation/baseline volume was used as the dependent variable. Descriptive statistics were calculated and their relationship analyzed using MATLAB 7.8.0 (MathWorks, Natick, Mass.) and the function “cftool.”
Table 1. Analysis of Six Published Articles Using Autologous Fat Transfer without Expansion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Results
Of the 84 women evaluated for enrollment in the study, three (3.6 percent) were turned away for failure to pass the Brava tolerance test in the office. We progressively increased graft volume as we became more comfortable with the procedure. The first 20 women were grafted conservatively with an average of 190 ml per breast, resulting in 90 percent graft survival, whereas the latest 20 were grafted an average of 360 ml per breast with 78 percent measured graft survival. Operating time for the first 20 cases averaged 4 hours and later decreased to 2 hours despite larger volumes as we developed the Lipografter to increase harvesting and grafting proficiency. There were no surgery-related complications. Average follow-up was 3.7 years (range, 12 to 75 months). Except for temporary bruising and superficial skin blisters that healed uneventfully, there were no significant complications, and all women returned to sedentary activities within 3 to 4 days and full activities within 1 week, with the liposuctioned donor sites as the only foci of morbidity. One patient developed a late (2 months postoperatively) atypical mycobacterial infection treated successfully with oral antibiotics and minor incision and drainage. Six women had unplanned pregnancies within 6 months after grafting. All had normal deliveries and breastfed. Follow-up magnetic resonance imaging scans were obtained 1 year after they stopped breast-feeding. None of the patients developed clinically suspicious breast masses or nodules. Although some women had minor weight fluctuations during the course of the study, the overall average body mass index did not change. All were very pleased with the enlargement and improved appearance of their breasts and liposuctioned donor sites (Figs. 3 through 5).
The 3- and 6-month magnetic resonance imaging scans were essentially unchanged (p > 0.4, paired t test), indicating that whatever graft survived at 3 months was stable. There were recognizable foci of fat necrosis in 12 of the 75 women. At 1 year, only these same 12 women (16 percent) showed some calcifications on mammography. All calcifications were clearly recognizable as benign fat necrotic foci. Because they were determined to be not suspicious for malignancy, they required no further intervention. Every focus of fat necrosis identified by magnetic resonance imaging was also recognized as a benign oil cyst by mammography, confirming that in this series, the 1-year mammogram was as sensitive as magnetic resonance imaging for the detection of fat necrosis. Because there was no change between the 3- and 6-month magnetic resonance imaging scans, the subsequently enrolled 47 women had only one magnetic resonance imaging scan at a minimum 6-month follow-up (average, 1 year). One of the 6-month follow-up magnetic resonance imaging scans was read as equivocal, requiring a repeated study 6 months later that confirmed the benign nature of the lesion.
Table 2 lists summary breast volumetric data of the 71 Brava-compliant autologous fat transfer–treated patients. The average volume of fat grafted was 282 ml per breast, with a resultant average augmentation of 233 ml per breast (range, 60 to 619 ml; SD, 108 ml per breast). Table 3 summarizes the published autologous fat transfer breast augmentation control series. Based on the available data (n = 124), the mean volume of fat grafted was 249 ml per breast, with a resultant weighted average volume augmentation of 134 ml per breast (range, 63 to 223 ml per breast; SD, 43 ml per breast). Statistical comparison of augmentation volumes achieved with Brava plus autologous fat transfer is significantly greater than the published series of autologous fat transfer augmentations (p < 0.00001, two-sample independent-variance t test).
The weighted mean graft retention rate of the published control patients (n = 335) was 55 percent, with a weighted SD of 18 percent. In our treated patients (n = 75), the mean graft retention rate was 78 percent (range, 0 to 129 percent). However, the mean retention rate for the treated compliant sample (n = 71) was 82 percent (range, 40 to 129 percent; SD, 18 percent) (p < 0.00001, two-sample independent-variance t test).
A dose-response curve illustrating the relationship between pregrafting Brava expansion (dose) and final breast augmentation (response) was developed. The expansion and augmentation data were normalized by dividing each variable by baseline volume, creating a ratio plotted in Figure 6. The correlation of determination (R2) between the two was initially derived using the linear least squares method. However, because there are several outliers in the data that weigh heavily on the fit, we used the “robust fit”3 method, which deemphasizes outliers to achieve an alternative fit. Figure 6 shows the robust fitted curve and its respective confidence interval boundaries.
Figure 7 illustrates the correlation between preoperative Brava expansion and augmentation volume. We subdivided the patients into four groups depending on their expansion ratio. Women who were not compliant and were poorly expanded could be considered as nonexpanded controls. They ended up with augmentation volumes comparable to the published autologous fat transfer series, whereas those who doubled or tripled their baseline volume as a result of Brava expansion achieved augmentation volumes comparable to moderate sized implants.
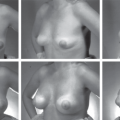
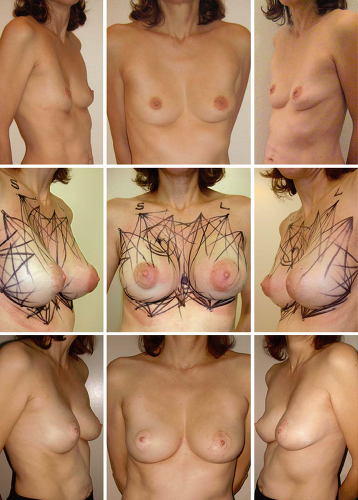 Fig. 3. Images of a woman with pectus deformity and asymmetry (above), showing maximal expansion just before fat grafting with the markings of the injection sites (center). Pectus and asymmetry have been corrected and stable augmentation has been achieved at 2.5-year follow-up (below).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|