36 Brachial plexus injuries
Adult and pediatric
Synopsis
 Complexity of brachial plexus injury (BPI): BPI is characterized by many complex problems and remains a dilemma to many reconstructive microsurgeons. These complexities include: (1) diverse injury patterns; (2) disrupted anatomy; (3) unpredictable nerve degeneration and regeneration; (4) difficult physical examination and diagnosis; (5) challenging nerve surgery; (6) long rehabilitation; (7) different palliative surgeries for sequelae deformity; (8) no consensus of outcome evaluation; and (9) difficult pain management. Many reconstructive microsurgeons show great interest but are greatly frustrated by this field.
Complexity of brachial plexus injury (BPI): BPI is characterized by many complex problems and remains a dilemma to many reconstructive microsurgeons. These complexities include: (1) diverse injury patterns; (2) disrupted anatomy; (3) unpredictable nerve degeneration and regeneration; (4) difficult physical examination and diagnosis; (5) challenging nerve surgery; (6) long rehabilitation; (7) different palliative surgeries for sequelae deformity; (8) no consensus of outcome evaluation; and (9) difficult pain management. Many reconstructive microsurgeons show great interest but are greatly frustrated by this field.
 Differences between adult and pediatric BPI: BPI can occur in adults and children. Although the anatomy is the same, there are many differences, including mechanism of injury, type and degree of injury, preoperative evaluation and diagnosis, surgical options, postoperative management and rehabilitation, palliative surgery for sequelae deformities, outcomes evaluation, and pain management (Table 36.1). Therefore, these two different entities will be discussed separately.
Differences between adult and pediatric BPI: BPI can occur in adults and children. Although the anatomy is the same, there are many differences, including mechanism of injury, type and degree of injury, preoperative evaluation and diagnosis, surgical options, postoperative management and rehabilitation, palliative surgery for sequelae deformities, outcomes evaluation, and pain management (Table 36.1). Therefore, these two different entities will be discussed separately.
Table 36.1 Differences between adult and pediatric brachial plexus injury
| Terminology | Adult brachial plexus injury | Obstetric brachial plexus palsy |
|---|---|---|
| Etiology | Trauma (more closed injuries than open) Traction injury, mostly by motorcycle accident | All are closed injuries Traction injury following delivery |
| Demography | All ages | All are infants and children |
| Physical examination | Complex (see text) | Difficult but simple (see text) |
| Horner’s syndrome | Reliable and persistent | Not reliable, not persistent |
| Spontaneous recovery by aberrant regeneration | Less | Many |
| Intraoperative findings | Level 1–4 injury Most are level 1 lesion | In operated cases, global > Erb’s palsy In nonoperated cases, Erb > global palsy All are supraclavicular injury Rarely associated with vascular damage Rupture injury, the gap is short (2–4 cm) (making the nerve regeneration by itself possible) Platysma, very thin or scarce |
| Surgical techniques | Nerve transfer ≥ nerve graft | Nerve graft ≥ nerve transfer |
| Postoperative immobilization | 3 weeks | 4 weeks |
| Rehabilitation | Good | Poor cooperation |
| Prognosis Nerve grafts in level 2 rupture injury, time to achieve elbow flexion >M3 C8 and T1 root injury C5 stump in four-root injury(C6–T1) Phrenic nerve transfer Intercostal nerve transfer Contralateral C7 transfer Oberlin transfer Intrinsic recovery following repair | Depending Usually takes 1 year All or none More healthy Quite strong and very often Fair results Often Often Never | High incidence of aberrant regeneration Usually takes 2 years Usually incomplete (especially T1) More impaired Risk for severe respiratory distress Usually good results Rarely applied Rarely applied Often |
Complexity of anatomy
Gross anatomy
Each spinal nerve is formed by the joining of the ventral root (motor fibers) and the dorsal root (sensory fibers). Each root is formed by a number of rootlets. The dorsal roots carry sensory information to the central nervous system, while the ventral roots convey motor fibers to the muscles. The cell bodies (neurons) of the motor fibers are located in the anterior horn of the spinal cord, while the cell bodies of the sensory fibers reside in the dorsal root ganglion located within the intervertebral foramen, immediately outside the dura mater of the spinal cord. The dorsal and ventral roots unite a few millimeters distal from the ganglion to form a spinal nerve, a mixed nerve, which goes through the interscalene space between the scalene anterior and middle muscles. Just out of the scalene muscles the five postganglionic spinal nerves make a first union to form the three trunks: upper (formed by C5 and C6), middle (C7 itself), and lower trunk (formed by C8 and T1 spinal nerves). Each trunk divides into anterior and posterior divisions just proximal to or directly under the clavicle. The nerves exchange fibers and form the second union, just distal to the clavicle, and are termed “cords.” Lateral and medial cords are anterior compartment nerves, passing anterior to the subclavian artery. The posterior cord is a posterior compartment nerve, passing posterior to the subclavian artery. The cords anterior to the subscapularis muscle run further distally behind the pectoralis minor muscle. Each cord has two or more terminal branches to the periphery (Fig. 36.1).
Disputed anatomy
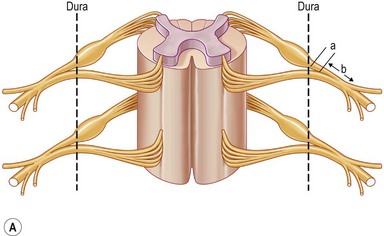
Various classifications of the level of BPI have been proposed: two levels of injury described by Leffert1 and Krakauer and Wood2; three levels by Terzis et al.3; four levels by Millesi,4 Alnot,5 and Chuang6; five levels by Narakas7; six levels by Mackinnon and Dellon8; and eight levels by Boome9 (Table 36.2). These numerous classifications have made the understanding of the anatomy of the brachial plexus complex and confusing. The most confusing aspect is the so-called postganglionic root (Fig. 36.2). Some anatomists term the part in the interscalene space before formation of the trunks to be “roots.”10 However, some call this part “spinal nerve.”11 In fact, after the dorsal root ganglion, both ventral and dorsal roots continue for only a few millimeters (<5 mm) in distance and unite to become a mixed nerve where it is no longer a root (Fig. 36.2). Sunderland11 has stated that “the term nerve root should be reserved for the paired anterior and posterior nerve roots in the spinal canal,” and that “the part of the plexus extending from the union of nerve roots to the formation of the trunks of the plexus should be referred to as a spinal nerve.” The author accepts this statement. Therefore, the components of the brachial plexus are roots, spinal nerves, trunks, divisions, cords, and terminal branches.
Table 36.2 Classifications for level of brachial plexus injury
| Authors | Levels | Area of injury |
|---|---|---|
| Leffert1 | 2 levels | Supraclavicular injury (supraganglionic, infraganglionic, and sub- or retroclavicular) and infraclavicular injury |
| Krakauer and Wood2 | 2 levels | Supraclavicular (roots, trunks and divisions), infraclavicular (cords and branches) |
| Terzis et al.3 | 3 levels | Root, supraclavicular postganglionic, infraclavicular |
| Millesi4 | 4 levels | Supraganglionic root, infraganglionic root, trunk, and cord |
| Alnot5 | 4 levels | Preganglionic root, postganglionic root, supra- and retroclavicular, and infraclavicular |
| Chuang6 | 4 levels | Preganglionic root, postganglionic spinal nerve, pre- and retroclavicular, and infraclavicular |
| Narakas7 | 5 levels | Supraganglionic root, infraganglionic spinal nerve, infraganglionic trunk, retroclavicular and terminal branches |
| Mackinnon and Dellon8 | 6 levels | Root avulsion (preganglionic and postganglionic), trunk injury, lateral cord, posterior cord, medial cord, terminal cord branch injury |
| Boome9 | 8 levels | C5–6, C5–7, C5–7 posterior division, C8–T1, C5, C6, lateral and medial cord, posterior cord |
Numerous anatomical variations of the brachial plexus do exist1,5 and should be always kept in mind. For example, the musculocutaneous nerve may sometimes arise from the median nerve and not from the lateral cord. In some rare cases of C5–6 root avulsion, the musculocutaneous nerve is still found to be functional because part of the musculocutaneous nerve derives from the median with its origins from C7.
Microanatomy
The microanatomy or internal topography of the brachial plexus has been extensively studied and described.12,13 The monofascicular pattern is usually found in regions of: (1) the spinal nerves; (2) anterior and posterior divisions of the upper trunk; and (3) the origin of the suprascapular and musculocutaneous nerve. Marked changes in fascicular topography occur every 10 mm. It is especially true at the level of the upper trunk where interfascicular crossovers are so extensive that direct repair or repair with short nerve grafts will frequently lead to co-contractions due to aberrant regeneration of a group of muscles. This aberrant regeneration is mainly found in obstetrical brachial plexus palsy (OBPP) patients but rarely in adult BPI. In addition, plexus connective tissue is more abundant than neural tissue. All these factors are reasons why the results of brachial plexus nerve surgery are so unpredictable. Knowledge of the internal topography of the plexus can be helpful in connecting corresponding nerve fibers with bridging nerve grafts. However, localization within the spinal nerve to define specific axonal groups to supply specific muscles or specific branches is difficult and not practical.
Anatomy with level of injury
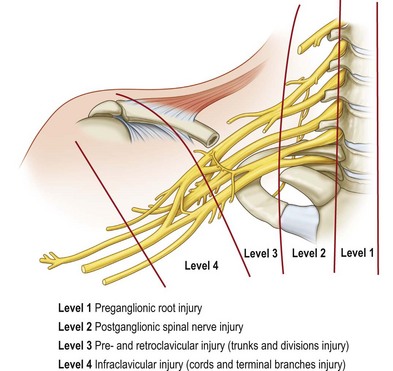
To avoid anatomical confusion, we have described brachial plexus lesions in terms of the level of injury 1–4 (Fig. 36.1). A total of 819 cases of adult BPI were included (1986–2003) in this new classification14 and the incidence at different levels was determined:
• Level 1 injury: inside the (vertebral) bone (preganglionic root) injury, including spinal cord, rootlet, and root injury – 70% (574 cases)
• Level 2 injury: inside the (scalene) muscle (postganglionic spinal nerve) injury, located at the interscalene space proximal to the suprascapular nerve – 8% (65 cases)
• Level 3 injury: pre- and retroclavicular injury, including trunks and divisions – 5% (45 cases)
• Level 4 injury: infraclavicular injury, including cords and terminal branch injury proximal to the axillary fossa – 17% (135 cases).
There are some relationships among the levels of injury:
1. An extended-level injury on the same nerve is frequently observed: for instance, C7 injury from the root level down to the interscalene space (level 1 and 2 injury).
2. A combined-level injury on different nerves is common: for instance, C5 and C6 spinal nerve rupture injury (level 2) accompanied with C7–T1 root avulsion (level 1).
3. A skip-level injury is rare: for instance, a longitudinal skip-level injury in which C5 and C7 are injured (avulsion or rupture) but C6 is intact; a horizontal skip-level injury in which level 1 and level 3 are injured, but level 2 is grossly intact.
4. Level 4 injuries are usually isolated, located infraclavicularly, and rarely show upward extension.
5. The term “supraclavicular BPI” will cover a large zone of injury, including level 1, 2, or 3 lesions.
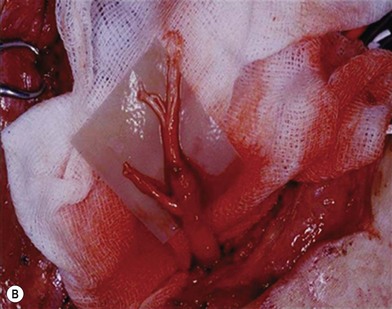
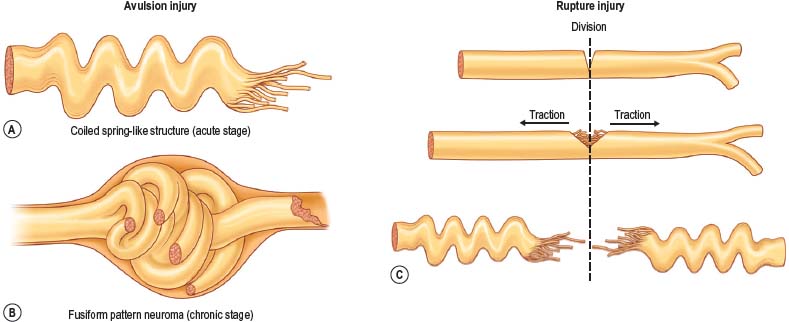
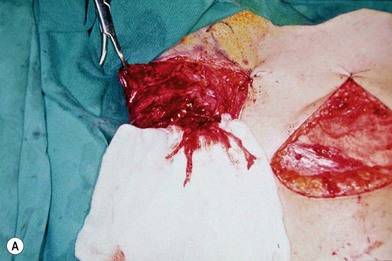
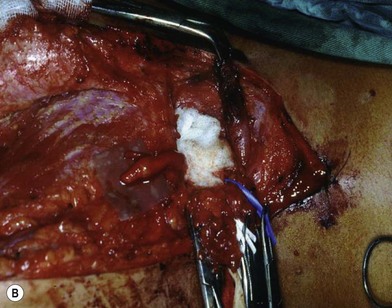
There are two types of characteristic lesions seen in BPI: avulsion and rupture (Fig. 36.3). Both are traction injuries but with different characteristics. Avulsion refers to the nerve being torn from its attachment (proximal avulsion occurs at the spinal cord, distal avulsion at the muscle). Rupture is a nerve injury involving a traction force on an incompletely divided nerve, causing a complete division with irregular proximal and distal ends (Fig. 36.3C). In avulsion injury, only one disrupted end with a coiled spring-like appearance can be seen in the operative field in the acute stage (Fig. 36.3A or Fig. 36.4A), or a fusiform pattern (glioma) in the chronic stage (Fig. 36.3B or Fig. 36.4B). If a surgeon attempts to locate the other disrupted end, a second operative wound is usually required. However, in rupture injury the two nerve ends can be visualized in the same operative wound in the acute stage, or within a big neuroma noted in the chronic stage.
Root avulsion is very common in BPI due to its weak supporting structures consisting of dura and dentate ligaments. A novel approach of performing spinal cord implantation with or without nerve graft15,16 showed unsatisfactory clinical results. This implies that in avulsion injury only one end (distal end) is available, while the other end (proximal) end is absent or unsuitable for repair. “Root injury” is an obscure term which may mean avulsion from the cord (true avulsion), or rupture or stretch at rootlets or roots. Root avulsion in BPI is usually accompanied by dura tearing and a cerebrospinal fluid leak with cyst formation, called pseudomeningocele. However in some cases the root can be avulsed at its origin with an intact dura cone (called “avulsion in situ”). The nerve root may remain inside the spinal canal or at the dural orifice, giving a grossly normal appearance or loosening with curvature of the spinal nerve at the time of surgical intervention despite established paralysis. Most often, however, the entire avulsed root, including ventral, dorsal roots, and ganglia, retracts and migrates downward to the interscalene or preclavicular region.
Pathophysiology and degree of nerve injury
Timing of nerve exploration is dependent upon the degree of nerve injury. The degree of peripheral nerve injury can be classified into neuropraxia, axonotmesis, and neurotmesis (Seddon classification17) or grade 1–5 injury (Sunderland classification18). Seddon’s axonotmesis or Sunderland’s second-degree injury starts to have wallerian degeneration at proximal and distal stumps. Seddon’s neurotmesis or Sunderland’s third- to fifth-degree injury has the potential for aberrant reinnervation after nerve regeneration. In Sunderland’s fourth- or fifth-degree injuries, only nerve repair can succeed in restoring continuity, but in first-, second- or third-degree injuries, spontaneous recovery, complete and incomplete, may occur.
There is rarely an argument for immediate exploration after penetrating injury by sharp objects for direct nerve repair. Some surgeons also advocate exploration of the BPI as early as possible19,20 for adult closed BPI for its advantages, including easy diagnosis of root avulsion and avoidance of difficult dissection through scarring. However, such early exploration is not recommended by most brachial plexus surgeons.2–6 In cases of closed BPI, the degree and extent of injury are difficult to judge soon after injury and are often underestimated. The benefits of waiting usually outweigh the advantages of early surgery.
Adult brachial plexus injury
Introduction
• Numerous etiologies can cause adult brachial plexus injury
• Clinical evaluation is still the most important step in establishing the site of injury, estimating the degree of injury and determining the surgical treatment and prognosis
• Imaging studies such as magnetic resonance imaging or computed tomography myelography are very helpful for diagnosing level 1 and/or level 2 injury
• Neurolysis, nerve repair, nerve grafts, nerve transfers, and functioning free muscle transplantation are all possible options for brachial plexus reconstruction. Different levels of injury have different reconstructive strategies
• There are many landmarks and key points for supraclavicular and infraclavicular brachial plexus dissection
• Brachial plexus reconstruction involves the use of palliative techniques for long-term sequelae
• Outcome evaluation should be unified to assess the success of the reconstructive technique and to report and share experiences which may help to improve current reconstructive techniques
Patient history
Patient history should include mechanism of injury, conscious level at the time of trauma, associated injury (such as head injury, fracture, open wound, chest injury, vascular injury), kinds of previous surgical intervention (such as chest intubation, cervical spine surgery), and characteristics of pain. This information helps to determine the degree and extent of injury and the need for surgical intervention. Mechanism of injury (e.g., upward or downward traction and with or without rotation) is not easily detected due to the patient’s loss of consciousness or amnesia for the accident. A history of shoulder dislocation or glenoid fracture may have a high incidence of level 4 injury, whereas a history of cervical spine injury or fracture may cause a level 1 root injury. Artery rupture and repair imply the site of nerve injury. For instance, arm traction by rolling machine or conveyor belt often causes an open wound in the axilla, extensive ecchymosis around the shoulder and chest (due to rupture of axillary vessels), and level 4 BPI. Segmental thrombosis of the subclavian artery is usually associated with C8–T1 root injury. History of rib fracture and chest intubation may preclude intercostal nerve transfer because of a higher failure rate.21 Extreme causalgia is often seen in cases of root avulsion in lower-root (C8–T1) avulsion as they contain the richest sympathetic fibers.22 Extreme causalgia is also a major factor for poor outcome due to poor rehabilitation.
Preoperative evaluation and diagnosis
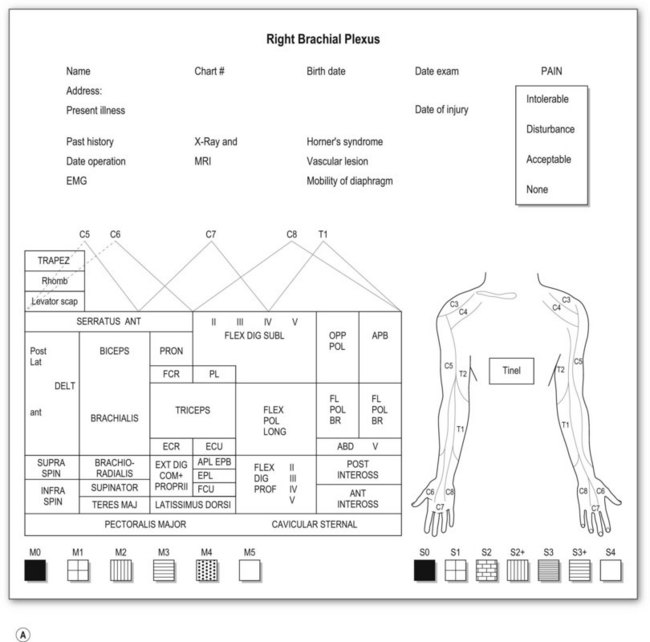
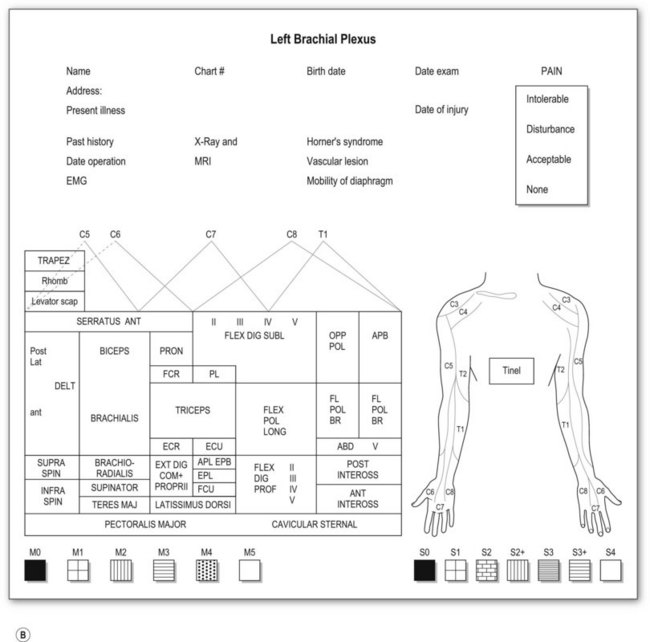
Most adult BPIs are closed injuries. Accurate assessment of the extent and severity of the injury in closed BPI is difficult. Clinical evaluation is still essential and is the most important step in establishing the diagnosis of site and degree of injury, and determining the treatment and prognosis. A brachial plexus chart (left and right formats, Fig. 36.5) outlining the possible injury should be completed before definite brachial plexus surgery. This chart is filled at the initial examination, usually performed at 2 months after injury. The chart is also useful for follow-up evaluations allowing comparison of clinical pictures.
Motor examination
Muscle by muscle examination should be completed in a distal-to-proximal fashion and recorded, using the British Medical Research Council (MRC) scale (M0–5).23 We have modified the motor evaluation system, adding more detailed differentiation: M5, strength against four fingers (examiner) resistance; M4, against one finger, resistance for longer than 30 seconds; and M3, against gravity (Table 36.3). M4 is recognized as useful muscle strength. The action of each muscle should be examined separately in relation to the movement of a single joint. Although there is no single muscle innervated by a single spinal nerve, some muscle palsy can give specific information related to the level of the injury. For instance,
1. Diaphragm palsy implies C4 and very proximal C5 (level 1) injury.
2. The levator scapulae muscle lies anterior to the trapezius muscle in the neck, and can be more easily detected than the rhomboid muscles, which are covered by the trapezius muscle. Both levator scapulae and rhomboid muscles are innervated by the same nerve (dorsal scapular nerve, or C4 and C5). Preservation of its function in upper plexus or total plexus injury may imply C5 rupture injury (level 2) after branching to the muscle.
3. Serratus anterior muscle: The long thoracic nerve has two portions: the upper portion originating from C5 and C6, and the lower portion from C7. The upper portion is responsible for scapular protraction, and the lower portion is important for scapular stabilization.24 Positive anterior traction of the scapula (shoulder protraction test) shows that at least C5 is ruptured after branching to the long thoracic nerve, so the proximal C5 is available for transfer. Scapular winging is observed only when the lower portion is denervated, but isolated C7 root avulsion is rarely seen in adult BPI. In pure C5–6 level 1 injury, the lower part of the muscle is still functional.
4. Clavicular and sternal portions of the pectoralis major muscle: The major pectoral muscle can be separated into two parts: clavicular and sternal parts. The clavicular part is innervated by upper and middle trunks or its divisions, while the sternal part is innervated by the lower trunk. A functional clavicular portion of the pectoralis major muscle may imply an infraclavicular (level 4) injury.
Table 36.3 British Medical Research Council (MRC) scale and Chuang modification
| British MRC scale | |
| Motor scale | Sensory scale |
| Chuang modification | |
| Motor scale | Sensory scale |
| M4 Active movement against examiner’s one-finger resistance ≥30 seconds | S2+ pain and touch with overreaction |
Sensory examination
Sensory evaluation should include sensory tests and elicitation of a Tinel’s sign. Sensibility tests include pain and temperature appreciation, static and moving two-point discrimination, constant touch, and vibration. However, performing complete sensory tests in BPI is both unnecessary and illogical because we are examining the dermatomal distribution from spinal nerves, not the cutaneous distribution. Pinprick test from areas of normal to abnormal sensation to map out the area of sensory disturbance is sufficient for most brachial plexus-injured patients. Sensory grading is based on the British MRC scale (S0–4),23 modified by adding a grade for sensory overreaction (S2+) (Table 36.3). Such sensory evaluations can give some clues about the level and degree of BPI.
Plain X-ray and imaging studies
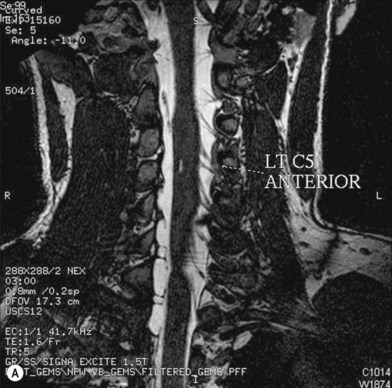
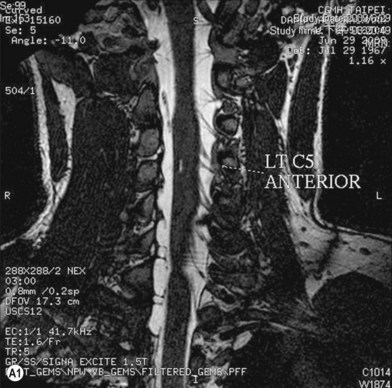
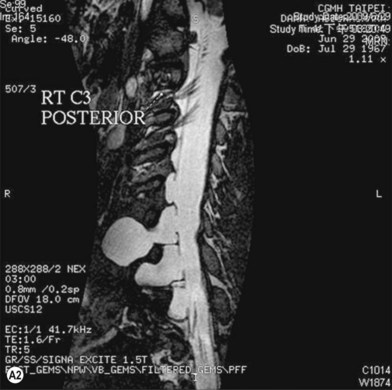
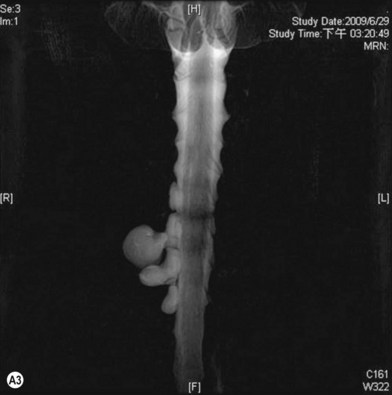
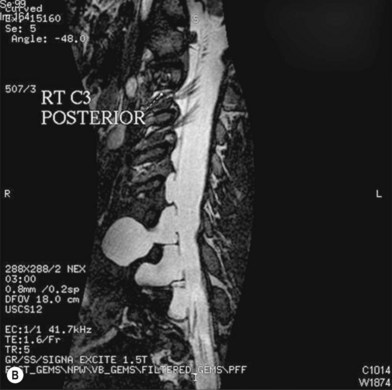
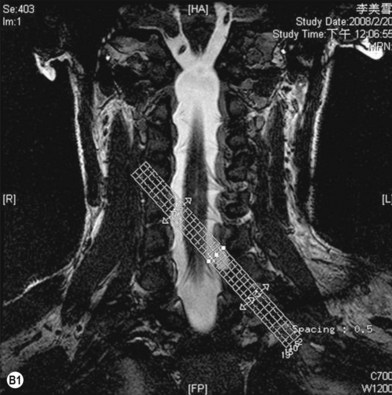
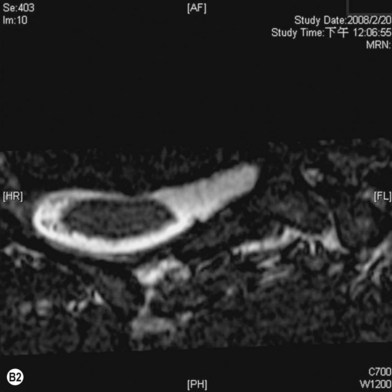
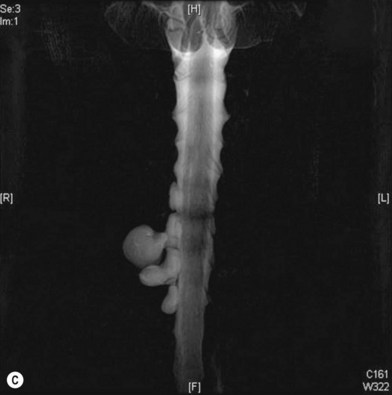
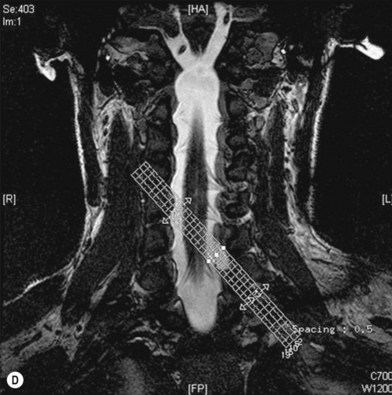
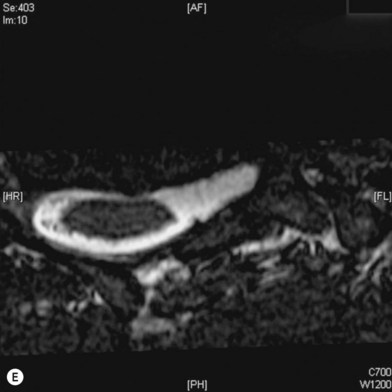
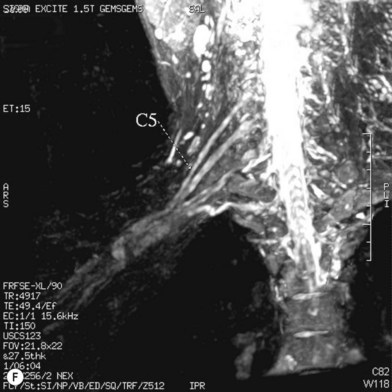
Cervical myelography and computed tomography (CT) myelography can provide valuable information related to the level 1 injury of the brachial plexus.25 However, in recent years these studies have been gradually replaced by noninvasive magnetic resonance imaging (MRI).26 The most useful MRI technique for the evaluation of a possible level 1 lesion is the three-dimensional (3D) fast imaging employing steady-state acquisition (FIESTA). These 3D source data are reconstructed along the planes of ventral and dorsal rootlets using a curve planar reformat technique to demonstrate the respective rootlets in a better perspective. Other MR techniques help in the imaging of the whole brachial plexus, notably of level 2 (Fig. 36.6).
Electrodiagnostic studies
Electrodiagnostic studies, mainly consisting of nerve conduction studies (NCSs) and needle electromyography (EMG), are used to localize the lesion and to assess its severity. For the NCS, only amplitudes of the sensory nerve action potentials (SNAPs) and compound muscle action potentials (CMAPs) are of value. Both SNAP and CMAP amplitudes provide a good indication of the degree of axon loss, or in contrast, the number of survival axons capable of conducting impulses.27,28 Sensory NCSs assess the function of the postganglionic portion of the sensory pathway. Therefore, abnormally low SNAP amplitudes indicate a ganglionic or postganglionic lesion. Conversely, SNAP amplitude remains normal in a pure preganglionic lesion such as root avulsion. A combination of unelicitable CMAPs with abnormal low SNAP amplitudes suggests a combined preganglionic and postganglionic lesion. To assess the major elements of the brachial plexus, NCSs of multiple nerves are usually required. These include sensory NCSs of the lateral antebrachial cutaneous, median, ulnar, radial, and axillary nerves, as well as motor NCSs of the median, ulnar, radial, musculocutaneous, and axillary nerves. The positive pick-up sensory action potentials taken from a paralyzed limb are evidence of preganglionic root lesion. The presence of fibrillation potentials on needle EMG may suggest that the lesion is at least axonotmesis. The reduction of amplitude of compound muscle action potentials during motor NCS is more reliable than the presence of fibrillation potentials to indicate axonal loss rather than a neurapraxia lesion.
Needle EMGs can detect a minimal amount of motor axon loss. For a comprehensive evaluation of the brachial plexus, adequate EMG sampling of muscles is critical. In addition to muscles innervated by major terminal nerves, examination of those supplied from or proximal to the brachial plexus can help to localize the lesion. These include the rhomboid major, serratus anterior, pectoralis major (both the clavicular and sternocostal parts), latissimus dorsi, teres major, and cervical paraspinus muscles. Needle EMG can also reveal evidence of early reinnervation and chronicity of the lesion. Presence of denervation potentials (i.e., fibrillation potentials and positive sharp waves) is the most sensitive indicator of motor axon loss. However, they require about 3 weeks to develop after axon injury.27
Surgical treatment and techniques
Level 1 injury: inside the (vertebral) bone (preganglionic root) injury, including spinal cord, rootlet, and root
The incidence of level 1 lesions is 70%. Nerves may be avulsed from the spinal cord (true avulsion), or ruptured at the preganglionic roots and rootlets. Partial Brown-Séquard syndrome is an example of true avulsion.22 One to all five roots may be avulsed. Root avulsion injuries lack central connection and are considered irreparable. Nerve transfer, pedicled muscle transfer, and FFMT provide the only possibilities for functional restoration.
Nerve transfer
Nerve transfer is a surgical option which intentionally divides a physiologically active nerve (with low donor morbidity) and transfers it to a distal, more important but irreparable paralyzed nerve. The procedure is best done within a golden time period, within 5 months of the injury,6 in order to reactivate the paralyzed muscle(s) effectively (aiming for M4). Nerve transfer can be broadly classified into four categories: (1) extraplexus; (2) intraplexus; (3) close-target nerve transfer; and (4) end-to-side neurorrhaphy nerve transfer.
Extraplexus nerve transfer
Extraplexus nerve transfer involves the transfer of a brachial plexus neighboring nerve (from either the ipsilateral or contralateral neck) to the avulsed brachial plexus for neurotization of a paralyzed nerve. The reported donor nerves in common use are mostly aimed at motor reinnervation.29 These donor nerves include the phrenic (Ph) nerve, spinal accessory (XI) nerve (accessed by an anterior neck approach), deep motor branches of the cervical plexus (cervical motor branches, CMB), hypoglossal nerve (XII), and the contralateral C7 (CC7) spinal nerve. Extraplexus sensory nerve transfer such as supraclavicular sensory nerve to median nerve transfer is sometimes used to provide sensation to the paralyzed hand.
Closed-target nerve transfer
Closed-target nerve transfer is a procedure that provides a direct coaptation at a more distal site, closer to the neuromuscular junction, thus achieving faster recovery of motor outcomes. Closed-target nerve transfer is defined as a procedure outside the supra- and infraclavicular fossa. Examples include spinal accessory (XI) nerve transfer to the suprascapular nerve via a posterior approach; partial ulnar nerve transfer to the biceps nerve; partial median nerve transfer to the brachialis nerve; long head of triceps branch transfer to the axillary nerve; intercostal nerve transfer to the biceps nerve, the musculocutaneous nerve, or the nerve of the long head of triceps; anterior interosseus nerve transfer to the radial or posterior interosseus nerve; and branch of the anterior interosseus nerve transfer to the deep motor branch of the ulnar nerve in the forearm. Closed-target nerve transfer is considered as distal nerve transfer.30 Selecting proximal or distal nerve transfer as a reconstructive strategy is now a subject of much debate (Table 36.4). Proximal nerve transfer (extraplexus and intraplexus nerve transfer) is traditionally still the main reconstructive procedure.
Table 36.4 Proximal nerve transfer versus distal nerve transfer in adult brachial plexus injury
| Proximal nerve transfer | Distal nerve transfer | |
|---|---|---|
| Philosophy | Traditional | New strategy |
| Donor nerve | Supraclavicular, far from the target muscle | The nearby nerve close to the target muscle |
| Advantages | Diagnostic and treatment Proximal nerve(s), powerful Nerve cut, fewer functional deficits | A treatment procedure, but not diagnostic No scars, easy dissection Shorter operation time Nerve-cut stumps: healthy, no scar Direct repair; no need to graft nerve Short rehabilitation, faster recovery |
| Disadvantages | More scars, difficult dissection Health of cut stumps is unpredictable May need long nerve grafts Longer operation time Longer rehabilitation period | Nerve cut, risk of causing deficits Risk of iatrogenic injury May need multiple incisions |
| Indication | All kinds of avulsion/rupture injury of brachial plexus injury | Not global injury After brachial plexus neurofibroma (C5 and C6) resection Intrinsic palsy of the hand |