Bipedicle Tram Flap Reconstruction
Kenneth C. Shestak
Overview
This chapter was written for the first edition of Surgery of the Breast: Principles and Art. It accurately reflected my thoughts about the bipedicle transverse rectus abdominus myocutaneous (TRAM) flap procedure and described the technical details of the operation as I performed it. My indications have changed somewhat and I use this procedure less than I previously did as described below. In addition I have made some modifications for closure of the abdominal wall donor defect as outlined. The following chapter reflects my current use of the bipedicle TRAM flap for unilateral breast reconstruction.
Transfer of the lower abdominal skin and adipose tissue to the breast produces the most consistently excellent restoration and reconstruction of the female breast from the standpoint of the aesthetic outcome and permanence. As detailed in other chapters of this book, the TRAM flap has evolved tremendously since its introduction by Hartrampf (1) in that it can be transferred as a single (2) or double muscle pedicle (3,4) flap based on the superior epigastric artery (SEA) or as a free microvascular flap (5,6,7) based on the deep inferior epigastric artery (DIEA) with only a small segment of rectus abdominis muscle or without any muscle at all in the form of a DIEA perforator flap (8). This microsurgical method of transfer provides a greater amount of blood supply to the subcutaneous adipose tissue in zone III and to a portion of zone II (the trans-midline tissue) (8). The past decade has witnessed a greater and greater number of surgeons who are confident and skilled with microsurgical tissue transfer and this has lead to the increased incidence of free tissue TRAM flap breast reconstructions.
The bipedicle method of performing the TRAM flap provides the surgeon with the maximal amount of well-vascularized lower abdominal tissue that can be brought to the breast for reconstruction in any given patient. As discussed later, my clinical experience has taught me that 90% of the adipose tissue between the umbilicus and pubic region (all of zone I and 80% of zone II on each side of the abdominal midline) can be reliably transferred when using it in the properly selected patient (9). The technique of splitting the muscles after locating the vascular arcade in each of the rectus abdominis muscles using a 20-MHz Doppler has not changed. Sparing the medial and lateral strip of rectus muscles and its overlying fascia (although neither is innervated) and attachments to the linea alba and linea semilunaris, respectively, provides additional security in donor-site closure.
Currently in my practice I use this procedure far less frequently. It now represents my technique of transfer in 3% of TRAM flap reconstructions compared with a frequency of 20% when the chapter for the first edition of this book was written. This because I believe that the technique of preliminary surgical delay of the TRAM flap provides vascularity to 25% of the immediately adjacent trans-midline zone (zone II), thus reducing the need to elevate both muscles in a considerable number of patients who were formerly treated with the bipedicle TRAM flap. Micro-surgical tissue transfer has a much more well established and prominent role in the transfer of this tissue. Whenever I do use the technique, it is always performed by a “split muscle harvest” or selectively elevating the central 50–60% of each muscle as guided by an ultrasonic Doppler location of the intramuscular vascular pedicle. In addition, I now use an onlay of synthetic mesh (Prolene) in essentially every patient in whom a “split-muscle” bipedicle TRAM flap” is used. The abdominal bulge rate in patients so treated is low, and reoperation for hernia repair has been virtually eliminated. Alternatively, I will perform a relaxing incision in the just lateral to the linea semilunaris line separating the external oblique fascia from its insertion into this anchor line (the linea semilunaris) thus allowing an much easier closure of the central musculofascial defect created by the harvest of both muscles. When there is absolutely no tension at the area of musculofascial closure whatsoever synthetic mesh is not required. I learned this from Dr Scott Spear.
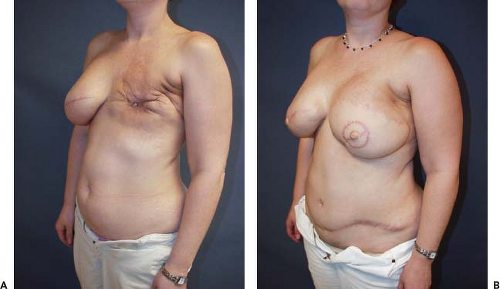
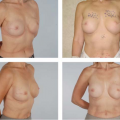
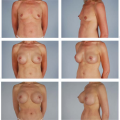
Currently the key indication for use of the bipedicle muscle pedicle flap for a single breast reconstruction in my practice is in the patient with a lower midline abdominal incision but who needs most or all of the tissue in the lower abdominal region for her reconstruction. Incorporation of both rectus muscles circumvents the effect of the midline scar’s interference with the circulation to the contra-lateral side of the abdominal adipose tissue and skin. The other indication is when all of the tissue in the lower abdomen is needed for the reconstruction. This is illustrated in the case example (Figs. 51.1A & B and 51.2A & B).
Thus although less common in its prevalence, the bi-pedicle procedure of transferring the TRAM flap using both rectus muscles as the vascular “carrier” remains an important option for the breast surgeon who has limited training and/or experience with microsurgical tissue transfer for the patient who requires more than 30% of the tissue on the non-pedicle side of a TRAM flap. As described in the following text, I have found that the split-muscle bipedicle TRAM flap is reliable, safe, and well tolerated by virtually every properly selected patient.
During the last 20 years, the TRAM flap has been firmly established as the standard for autogenous tissue breast reconstruction worldwide. It provides the reconstructive surgeon with the ability to simulate a breast of almost any size and shape while simultaneously improving the contour of the lower abdominal flap donor area. Although it was initially described as a unipedicle island flap by Hartrampf (1,2) the tissue of the lower abdomen also can be transferred as a bipedicle (double-muscle) flap. The procedure can be performed as a microvascularly augmented unipedicle or “supercharged flap,” and it may be done as a free-tissue transfer or free flap. The choice of flap elevation technique when using the TRAM flap is determined most importantly by tissue requirements for the breast reconstruction along with the aesthetics of the contralateral
breast and the experience and confidence level of the surgeon with the various options.
breast and the experience and confidence level of the surgeon with the various options.
Tissue requirements relate to the amount of skin and subcutaneous fat needed to reconstruct a breast form that will best approximate the contralateral breast. Careful assessment of this volume requirement is an essential first step in consistently achieving good outcomes with TRAM flap breast reconstruction. Simultaneous with this, assessment of the amount and distribution of adipose tissue on both sides of the abdominal midline is noted. The determinants of circulation to the superiorly based TRAM flap (10) include flow through the superior epigastric vascular pedicle, the number and flow through the musculocutaneous perforating vessels, flow across the midline, and venous outflow from the flap. The arterial supply of the lower abdominal skin and subcutaneous fat was initially investigated by Taylor et al (11). Via dye injection studies, they showed that the blood supply was derived from three sources, namely the deep inferior epigastric artery, the superior epigastric artery, and the intercostal arteries, with the deep inferior epigastric artery being the dominant pedicle. Concepts about the circulatory dynamics of the superiorly based TRAM flap were initially proposed by Hartrampf (1) after an analysis of his clinical experience. His observations gave rise to the idea of dividing the tissue of the lower abdomen into four zones (Fig. 51.3) based on the proximity of tissue in each area to the muscle pedicle. He observed decreasing predictability in survival of tissue as one progressed from zone I, the tissue overlying the pedicle, to the random pattern circulation of zone IV.
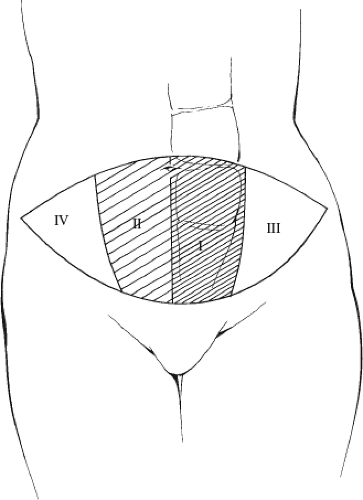 Figure 51.3. Designation of tissue zones (I through IV) based on vascularity to the different areas of the TRAM flap. |
As alluded to above the mechanics of circulation in the superiorly based unipedicle TRAM flap was further elucidated by Moon and Taylor (10) in another series of injection studies performed in cadavers. This work demonstrated a stepwise decrease in blood flow through “choke vessels” and gave additional support to the clinical observations of Hartrampf and Wagner et al. (4) and Bostwick (3). In a subsequent publication, outlined the “safe zones” of the unipedicle TRAM flap, which included approximately 2.5 zones of tissue (Fig. 51.4). These were represented by all of zone 1, 60% of zone II, and 70% of zone III. The investigators pointed out that if additional tissue was required to perform a breast reconstruction, then an increase in blood supply was necessary if the complications of fat necrosis and vascular compromise were to be kept to an acceptably low range. They stated that frequently it was necessary to perform a bipedicle flap for a unilateral breast reconstruction.
The venous circulation of the unipedicle flap was studied by Carramenhae et al (12) and Taylor (13). Their injection studies demonstrated that the veins of the flap accompanied the arteries within the muscle but that there were valves in connecting veins that were oriented inferiorly such that reversal of flow in these “oscillating” veins must occur if venous outflow in the superiorly based flap is to progress normally.
My clinical experience over the last decade with TRAM flap breast reconstruction has suggested that a superiorly based unipedicle flap will predictably support viability of two zones of tissue. More specifically, I believe that zone I, 80% of zone III, and 20% of zone II will consistently be carried by a single muscle pedicle when both the medial and lateral rows of perforators are incorporated with flap elevation. If tissue requirements exceed this amount of tissue volume, then additional vascular supply must be provided for the flap to minimize the risks of fat necrosis and flap loss.
Maneuvers to increase the circulation include designing the skin paddle as “midabdominal TRAM” (14), preoperative delay of the flap by ligation of the superficial inferior epigastric and deep inferior epigastric vessels 2 weeks before surgery (15), flap transfer with a microvascular augmentation or “supercharging” maneuver (16), free microvascular flap transfer (5,6,7), or performing a bipedicle flap incorporating both rectus muscles as vascular carriers (3,4). The bipedicle TRAM flap allows the transfer of virtually all of the abdominal tissue (Fig. 51.5) to the chest region. In the majority of procedures requiring more than two zones of tissue, I have used a split-muscle bipedicle flap with highly satisfactory results and a low complication rate in the breast and abdominal regions.
Patient Selection
Both patient selection and procedure selection (technique of flap transfer) are critical determinants of consistent success with TRAM flap breast reconstruction. A careful history and physical examination are performed, focusing on known risk factors that predispose the patient to increased complications with TRAM flap reconstruction (17). Most important, these include smoking, previous abdominal surgery and abdominal scars, prior radiation therapy, the presence of significant systemic disease, and pronounced obesity (17). Patients who smoke are asked to stop completely for 6 weeks before surgery. The presence of previous incisions, especially a right subcostal incision for a cholecystectomy and pronounced obesity in the
setting of the need for more than 2.5 zones of tissue often prompts the selection of a procedure different from the split-muscle bipedicle TRAM flap, and most commonly the free TRAM operation is used in these higher-risk situations (18,19).
setting of the need for more than 2.5 zones of tissue often prompts the selection of a procedure different from the split-muscle bipedicle TRAM flap, and most commonly the free TRAM operation is used in these higher-risk situations (18,19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree