and Emir Q. Haxhija2
(1)
Department of Plastic Surgery and Burns, Institute for Mother and Child Health Care of Republic Serbia, University of Belgrade, New Belgrade, Serbia
(2)
Department of Pediatric and Adolescent Surgery, Medical University Graz, Graz, Austria
Keywords
Benign soft tissue tumorsChildrenTreatment11.1 Introduction
Pediatric tumors are highly varied in origin and clinical presentation [1]. Benign skin and skin-associated soft tissue tumors can be classified simply into those that are epithelial, cutaneous appendage, neural crest, or mesenchymal origin and inflamatory lesions [2, 3]. From radiological point of view regarding imaging features, they can be divided into tumors of the epidermis and dermis, tumors of subcutaneous tissue, and tumors associated with fascia overlying the muscle, and there are also metastatic tumors, other tumors and tumorlike lesions, and inflamatory lesions [3]. Pediatric masses can have their origin due to errors along the pathways of embryological development [2]. Possibility that benign tumors can have malignant alteration has always to be considered [2, 4, 5].
Pediatric skin and soft tissue tumors are not uncommon [1, 2, 5–9]. History and thorough clinical examination, child age at presentation, location of the tumor, and rate of growth are essential diagnostic criteria [4, 6–8]. Clinical examination must asses the site, number of lesions, consistency (shape, size, elevation status, surface status, color, hardness, alignment), mobility, subjective symptoms of patient, and the time course of the appearance of the lesion [1, 4, 8]. The imaging of soft tissue tumors can be unspecific, and radiography, ultrasonography (US) including Doppler, computerized tomography (CT), and magnetic resonace imaging (MRI) usually provide much information about the characteristics of a mass [1, 3, 4, 8]. Benign and malignant tumors can be difficult to distinguish in children [4, 6]. If diagnostic procedures are not adequate to establish the diagnosis, after careful inspection and palpation, the biopsy should be performed [1, 4, 8]. Diagnosis and management of cutaneous defects that occur during embryogenesis are very difficult, and they require appropriate physical exam, laboratory testing, and often imaging studies [9].
11.2 Developmental Defects
11.2.1 Cranial Defects
Midline diverticulum of the dura normally projects anteriorly (fonticulus frontonasalis) and anteroinferiorly (prenasal space), and they closed during normal development leaving frontonasal suture and foramen cecum [2, 10]. If this dural diverticulum stays adherent to ectoderm, anomalies such as dermoid and epidermoid cyst, nasal dermal sinus, nasal gliomas, and cephalocele may occur [10]. Cephaloceles are persistent dural diverticula containing intracranial contents that herniated through the cranial defect [9, 10]. Meningocele contains meninges and cerebrospinal fluid, while protrusion of meninges and brain tissue through skull defect is called meningoencephalocele [9]. Heterotopical glial tissue is the presence of brain tissue in the scalp without an open cranial defect [9, 11]. Imaging studies for cranial defects include US, MRI, and CT, and if occult central nervous system (CNS) connection is identified, neurosurgeon consult should be obtained [2, 9].
11.2.2 Nasal Defects
Tumors in this region are uncommon, and they are not allowed to be excised or biopted before the diagnostic is performed [9]. Ultrasound can determine characteristic of the lesion (solid or cystic), and MRI is the diagnostic tool of choice to determine connection of skin or subcutaneous lesion with CNS [9, 10, 12–14]. Treatment of lesions in this region requires multidisciplinary approach [9–15].
11.2.2.1 Nasal Dermoid Cyst and Sinus
Nasal dermoid cyst and sinus (NDCS) is a rare developmental anomaly with an incidence of 1:20,000 to 1:40,000 births that can be present as cyst, sinus, or fistula and may have intracranial extension in 4–45% [10–14]. Pathogenesis involves the incomplete obliteration of neuroectoderm in the developing frontonasal region [2, 12]. It is mostly present as nasal dorsum mass (79%) and may be associated with sinus opening [2, 12–14]. Intermitent discharge of the sebaceous material and recurrent infection are common, with hair protruding through a punctum as a pathognomonic sign [12]. Progressive enlargement of a nasal dermoid can cause soft tissue and skeletal deformity, local infection, meningitis, and brain abscess [12]. Different imaging modalities, such as CT and MRI are used to determine extent of the lesion and intranasal or intracranial extension [13, 14]. There is a classification of nasal dermoids into superficial, intraosseous, intracranial extradural, and intracranial intradural [14]. On histologic exam, nasal dermoid is characterized as well-defined cyst lined by squamous epithelium of ectodermal origin with adnexal structures of mesodermal origin [12, 13].
Complete excision is the treatment of choice (Figs. 11.1a–f and 11.2a–d) [12, 13]. If there is no sinus opening in the skin, open rhinoplasty approach is recommended, and if there is sinus tract opening, it must be excised (with small excision) along with sinus tract which is excised through open rhinoplasty approach or endoscopic approach [2, 13]. In case of sinus extension deep to the nasal bones, nasal osteotomy is recommended in vertical manner so the tract can be followed proximally [2, 12, 13]. When intracranial extension is present, first, the extracranial approach is performed, and stalk of visible tract is sent to a frozen biopsy, and if it is positive, the intracranial approach is performed via bicoronal incision (“keystone” approach) or via endoscopic endonasal skull base surgery (EESS) [12, 13].
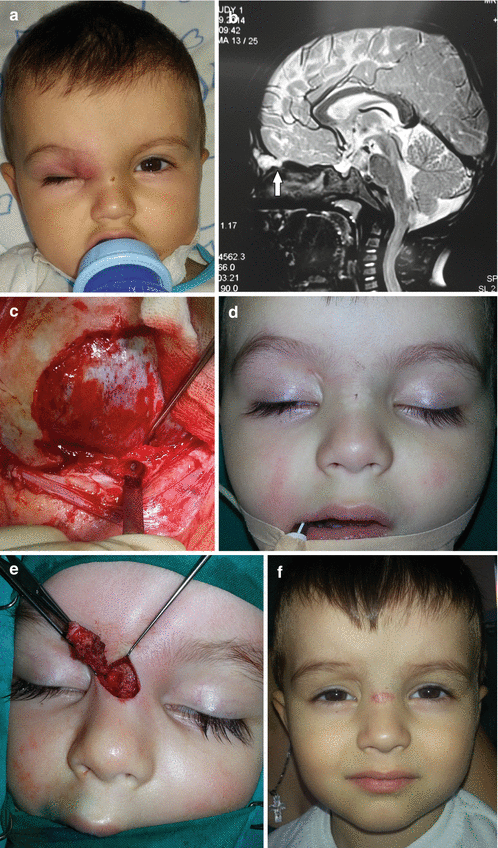
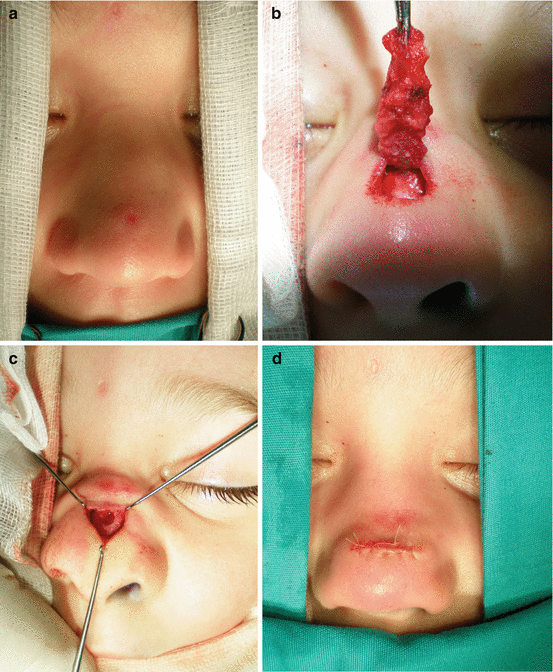

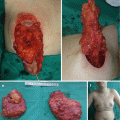
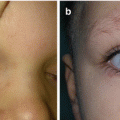
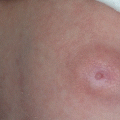
Fig. 11.1
Nasal dermoid cyst and sinus with intracranial propagation: (a) inflammation and edema of the right orbital region; (b) magnetic resonance imaging revealing intracranial propagation; (c) intraoperative finding (bone defect); (d and e) secondary surgery performed since there was rest of the extracranial part of sinus; (f) the result 3 months postoperatively

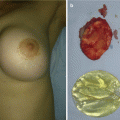
Fig. 11.2
Nasal dermoid sinus excision: (a) sinus opening at nasal dorsum; (b) intraoperative view; (c) complete excision of the sinus; (d) postoperative view
11.2.2.2 Nasal Glioma (Nasal Heterotopic Glial Tissue)
Nasal glioma is a rare congenital malformation that presents mature brain tissue isolated from the cranial cavity or spinal canal [9, 11, 15]. It is derived from either entrapped neuroectodermal tissue or a nasal encephalocele which later becomes disconnected [11, 15]. Imaging studies such as CT, MRI, and nasal endoscopy are used to differentiate nasal glioma from encephalocele (biopsy or aspiration is contraindicated) [11]. The treatment of choice is surgical excision (for intranasal glioma, transnasal endoscopic approach is recommended) (Fig. 11.3a, b) [11, 15].
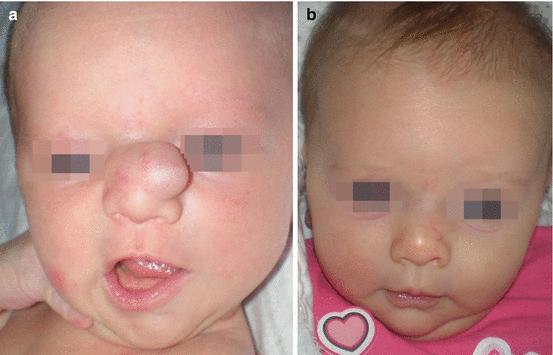

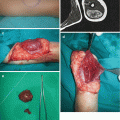
Fig. 11.3
Nasal ectopic glial tissue excision: (a) lesion of the left nasal region; (b) postoperative result
11.2.2.3 Dermal Sinus Tracts
Dermal sinus tract (“ectodermal inclusion cysts”) is result of incomplete sequestration of the neuroectoderm and somatic ectoderm, and it can occur from the occiput to sacrum [2, 16, 17]. They are most commonly presented with hypertrichosis, skin tags, and abnormal pigmentation [1]. MRI is the main diagnostic tool, and treatment is surgical (Fig. 11.4a–c) [1, 2, 16, 17].
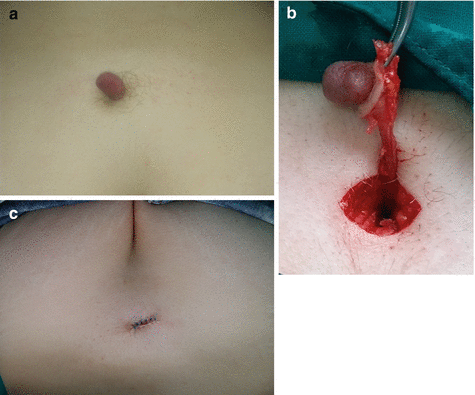

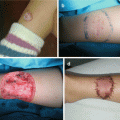
Fig. 11.4
Dermal sinus tract excision: (a) clinical appearance; (b) excision of the sinus tract; (c) postoperative view
11.2.3 Sinus Preauricularis and Accessory Tragus
These are common anomalies presented as an epithelial-lined sinus “pit,” skin tag, or a skin tag containing cartilage (“accessory tragus”) [18]. Accompanied anomalies of urinary tract are present in 8.6% of the patients with these anomalies, and US of urinary tract should be performed [9]. The treatment is surgical because of aesthetic reasons in case of accessory tragus and to prevent infections and further complication for sinus (Fig. 11.5a–d) [9, 18].
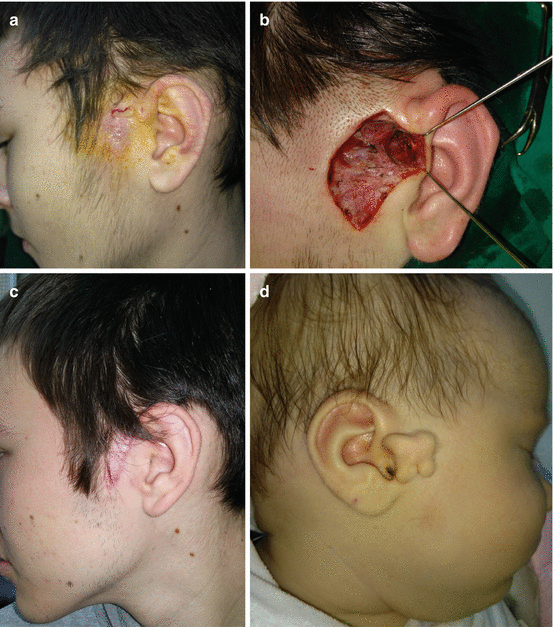

Fig. 11.5
Excision of preauricular sinus: (a) inflammation of preauricular region; (b) complete excision; (c) postoperative result; (d) accessory tragus
11.2.4 Neck Anomalies
Pediatric neck masses present diagnostic and therapeutic challenge, requiring extensive knowledge of the anatomy, the embryology, and the pathology [10, 19–30]. In children, most lesions are benign, either congenital or inflammatory [20].
11.2.4.1 Thyroglossal Duct Cyst
The thyroid gland develops during third gestational week (GW) in form of ventral diverticulum from the endoderm of the first and second branchial pouches [2, 26]. As the median thyroid anlage descends caudally, a tract forms the thyroglossal duct [23, 24, 26]. Thyroglossal duct typically involutes between 7 and 10 GW, and thyroglossal duct cyst (TGDC) remnant is formed if the tract persists or fails to obliterate, along any portion of the thyroid descent (base of the tongue and pyramidal lobe of the thyroid [2, 23, 24, 26].
TGDC anomalies are the most common congenital anomaly of the neck and the second most common neck mass found in children (50% is manifested until 10 years of life) [19, 23, 24, 26]. TGDC remnants occur in approximately 7% of population and both sexes are equally affected [24, 26]. TGDC is most commonly located ahead of thyrohyoid membrane and in suprahyoid region, but it can also be located anywhere from the base of the tongue to thyroid gland [2, 26]. The cyst is always primary anatomical presentation and the sinus is secondary [2].
Clinically, in most cases, there is asymptomatic, cystic neck mass, and near one third of patient is presented with infection, and one forth is presented with draining sinus (incorrectly used term fistula) [2, 23, 24, 26]. Lateralization of TGDC can occur in 10–16% of cases [23, 24]. Histologically, TGDCs are lined by respiratory and/or squamous epithelium [20, 24]. It is important to rule out the ectopic thyroid gland (sometimes the TGDC can occur within the thyroid gland) [24]. Malignant alteration is present in 1% of cases (papillary thyroid carcinoma, squamous cell carcinoma) [19, 23, 24].
Imaging includes US, CT, or MRI, and sometimes thyroid scintigraphy and screening of thyroid-stimulated hormone TSH are indicated [23, 26, 30]. Differential diagnosis includes dermoid cyst, epidermoid cyst, lymph node, lipoma, and thyroid adenoma [23, 24, 26, 30].
Treatment is surgical excision by the Sistrunk procedure [2, 23, 24, 26]. After identification of cyst, the channel is followed up to the hyoid bone, excision of the hyoid bone is performed, and the tract is ligated near its proximal origin (Fig. 11.6a, b) [2, 23, 30]. Complications of the procedure include bleeding, hematoma, and infection [23, 24]. Recurrence is reported in range of 2.6–5% (and up to 47%), and “extended” Sistrunk procedure should be performed in that case [2, 19, 22, 24, 26, 30]. There are also reports of successful treatment of TGDC with 99% sterile ethanol [21].
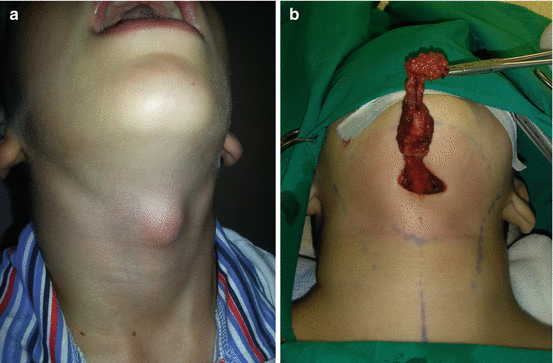

Fig. 11.6
Thyroglosal duct cyst excision: (a) preoperative view; (b) complete excision by Sistrunk procedure
11.2.4.2 Branchial Arch Anomalies
Branchial arch anomalies (BAA) present 20–30% of the excised cervical masses in children, and they are the most common lateral neck masses [19, 26]. Branchial anomalies (BAs) can present as cyst (80%), sinus, or fistula, and they result from the maldevelopment of the branchial apparatus which consists of grooves-ectoderm, arches-mesoderm, and pouches-endoderm during the embryonic period [2, 26, 27, 30]. BAs are typically present in infancy and childhood, but it can be diagnosed for the first time at any age with males and females equally affected [19, 27, 28, 30].
Diagnosis is made in more than half of cases by anamnestic data and physical examination, and sometimes additional radiographic studies are needed [9, 27]. On histology, first arch BAs are lined with squamous epithelium, and the second and third arch anomalies are composed of squamous and respiratory epithelium [19, 27, 29, 30]. US, CT, MRI, fistulography, and direct laryngoscopy are the diagnostic tools used for BAAs, and definitive treatment is surgical [19, 27–30].
Second BAAs present 70–95% of all BAs [2, 9, 19, 26, 28, 30]. The second BA forms the hyoid bone and the adjacent structures, and the second pouch-endodermal layer forms the epithelium of palate tonsil and the supratonsillar fossae [19, 26]. The majority of SBAAs are cyst (divided into four types by Bailey) [30]. Treatment of the second BAA cysts is surgical (Fig. 11.7a–c) [27, 28]. Second BAA can form the tract from supratonsillar fossa to the skin on anterior border of the sternocleidomastoid muscle (SCM) [19, 26]. The fistulae of second BA are typically presented at infancy or childhood, and cysts are presented in older age, mostly as nontender, soft mass, deep to anterior border of SCM, manifested after respiratory infection [2, 9, 26]. Excision of fistula can be performed through single or through stepladder incision (Fig. 11.8a–c) [19, 26–28].
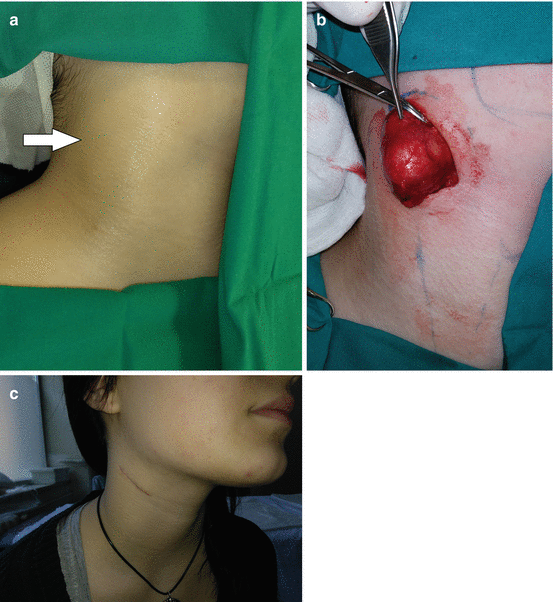
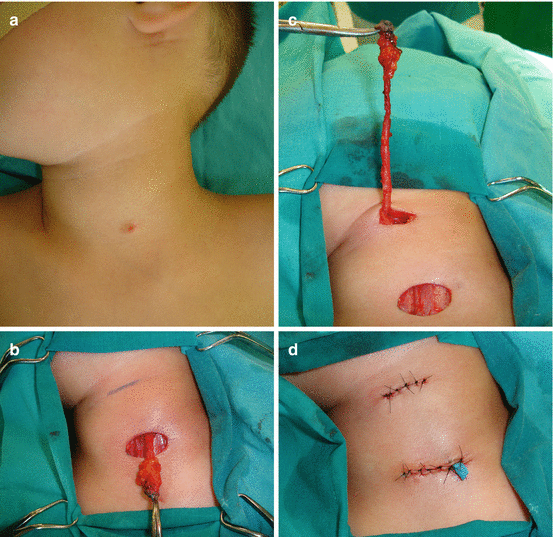

Fig. 11.7
Second branchial arch cyst excision: (a) preoperative view of the right lateral neck region; (b) excision of the cyst; (c) acceptable scar 2 months postoperatively

Fig. 11.8
Second branchial arch fistula excision: (a) preoperative view (fistula opening at left side of the neck region); (b) complete excision of the fistula through stepladder excision; (c) postoperative view
First BAAs present 1–8% of branchial cleft anomalies; they are located in proximal part of the neck in parotid region or submandibulary, always superior to the hyoid bone, mostly presented as cysts [2, 18, 19, 26]. Belanky and Medin divide first BAAs into type I, with the tract passing laterally or superiorly to the facial nerve without opening into the external auditory canal and type II with the tract passing superiorly or superomedially to the facial nerve (inconsistent relationship) with opening into external auditory canal, and there is also classification of first BAAs proposed by Work and Arnot [19, 26, 28, 30]. Preauricular cyst and sinus can be misdiagnosed for first BAAs [18]. Treatment of these anomalies is surgical and challenging because of their proximity to the facial nerve (facial nerve palsy is described in 10–40% of cases) [1, 19, 26–28].
Third and fourth BAAs are rarest among brancial arch anomalies with cranial origin at pyriform sinus and with close proximity with the thyroid gland at inferior part [2, 19, 26, 29, 30]. Third and fourth branchial arch anomalies are presented at any age, usually as lateral neck mass or suppurative thyroiditis, and in most cases on the left side [19, 28–30]. They are differentiating by their relationship with the superior laryngeal nerve and common carotid artery, by their opening at the pyriform sinus, and by the presence of thymic and thyroid tissue [28, 29]. Diagnostic tools include US, barium swallow, CT, and MRI [2, 27, 28, 30]. Complete excision is golden standard with chemocauterization of pyriform fossa sinuses and with hemithyroidectomy in some cases [26, 28–30]. There are also reports of successful treatment by sclerosation of BA cysts with OK-432 [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree