Melanocytic lesions are of importance primarily because of malignant melanoma, which is the single most common potentially lethal neoplasm of the skin. The incidence of melanoma has risen dramatically over the last several decades. However, the mortality has risen less dramatically than the incidence, likely due to earlier diagnosis. There is considerable geographic variation in the incidence of melanoma, related to exposure to sunlight and the susceptibility of the population. Thus, the global incidence is highest in the Australian tropics, but very low in most other tropical countries with their less susceptible populations.
Benign melanocytic nevi occur in all ethnic groups, although there is evidence for shared etiologic factors with melanoma, with a greater incidence in sun-susceptible populations living in sunny climates. Nevi and other benign pigmented lesions are occasionally of cosmetic significance, especially in the case of giant or so-called “garment” congenital nevi. Other than being cosmetically important, nevi are of importance primarily in relation to melanoma. Thus, nevi are important as simulants of melanoma, as potential precursors of melanoma, and as risk markers of individuals for development of melanoma. Because nevi are simulants of melanoma both clinically and histologically, criteria that distinguish between nevus and melanoma are of critical importance. This differential diagnosis, especially at the histologic level, is the primary topic of this chapter.
PRINCIPLES OF MANAGEMENT OF PIGMENTED LESIONS
As has been recently reviewed (1), there is discordance in the histologic diagnosis of melanoma, resulting in diagnostic uncertainty and complicating decision making for appropriate treatment (2,3). This occurs because of complexity in the histologic continuum from benign to unequivocally malignant melanocytic lesions, and is most pronounced in the gray zone between wholly benign and obviously malignant lesions (4). The difficulties are likely to be less pronounced within a single institution where patterns of diagnostic terminology and communication have been established. Subjectively, this variation in terminology appears to be especially troublesome between institutions that adhere to different schools of thought. Between institutions, and especially when there is variation in expertise and experience, the level of disagreement can be high (5).
This lack of standardization is not unique to melanocytic pathology, but affects other clinical fields. To improve precision in breast imaging, BI-RADS (Breast Imaging Reporting and Data System) was developed by U.S. Food and Drug Administration mandate and under the auspices of the American College of Radiology. This system standardizes results of mammogram interpretations and reports along a 5-point scale, with the objective of minimizing ambiguity regarding the necessity and type of therapeutic management. A similar system is under development for the melanocytic tumor system as the MPATH-Dx (Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis) schema that comprises a Histology Reporting Form and a Diagnosis–Treatment Mapping Tool (1). The terms used in the Histology Reporting Form are the “diagnoses” that are discussed in this and other reference texts and literature. The impact of variation in the exact form of words used for diagnosis in different classification systems is lessened when management implications are included as a primary outcome of the diagnostic process. The Mapping Tool is essentially a thesaurus that aims to encompass all of the terms in current use and maps these to one of five M-Path Dx categories. These are intended to represent possible clinical approaches for management of a lesion that is assumed for this purpose to be present at a specimen margin. The approaches vary according to the anticipated degree of aggressiveness believed to be associated with each diagnostic category, as follows (1):
MPATH-Dx Category 0: Incomplete study due to sampling and/or technical limitations. Clinical Outcome: Repeat testing or short-term follow-up.
MPATH-Dx Category 1: Benign lesions with essentially no probability of adverse consequences; for example, common (including mildly dysplastic) nevi, lentigines, and similar disorders. Clinical Outcome: No further treatment required.
MPATH-Dx Category 2: Lesions for which the probability of progressive disease is considered unlikely, but some risk for continued local proliferation and possible future adverse consequence cannot be ruled out; for example, some examples of Spitz tumors, deep-penetrating nevi, moderately dysplastic nevi. Clinical Outcome: Consideration of narrow but complete excision (<5 mm).
MPATH-Dx Category 3: Lesions with a higher likelihood of local tumor progression and greater need for intervention; for example, melanoma in situ, most examples of severely dysplastic nevi. Clinical Outcome: Excision with at least 5 mm (but <1 cm) margins.
5. MPATH-Dx Category 4: Lesions with substantial risk for locoregional progression; for example, invasive melanoma, American Joint Committee on Cancer (AJCC) Stage T1a. Clinical Outcome: Wide excision (≥1 cm margins).
6. MPATH-Dx Category 5: Lesions with greater risk for locoregional progression; for example, invasive melanoma, AJCC Stage Tb, or more. Clinical Outcome: Wide excision (≥1 cm margins); consider sentinel node staging, possibly other adjuvant therapy.
Substantial health care resources are directed to the screening of populations considered to be at increased risk of melanoma, as in other neoplastic systems. Ambiguities in diagnostic reports are common, and there is a tendency to electively treat ambiguous lesions. Screening studies have lead to a greater number of diagnoses of melanoma, without a concomitant increase in mortality, leading to suggestions that melanoma, like other cancers, is being overdiagnosed at a biological level, even in cases where there is good agreement as to a given diagnosis (6). This is because there are presumably many cases that would never have progressed to clinical malignancy. Present technology does not provide the tools to distinguish the harmless lesions from those that would have progressed if not excised, although developing sophistication in genomic studies such as fluorescence in situ hybridization (FISH) has the potential to improve this situation (4). Harm to patients can result not only from undertreatment but also from overtreatment of ambiguous disease in the forms of false fear of cancer, of morbidity from unnecessary treatment, and of misdirection of health care resources. These issues result not only from intrinsic limitations of the diagnostic processes but also as a response to medicolegal pressures and patient safety concerns. The MPATH-Dx Diagnostic–Treatment Mapping Tool may serve as an aid in reducing uncertainty and ambiguity in melanocytic lesion reporting and allowing for greater consistency in the management of pigmented lesions, and also by facilitating the study of outcomes and resource allocation at the level of populations and health care systems (1).
BENIGN MELANOCYTIC PROLIFERATIONS
Proliferations of melanocytes are usually pigmented and are often synonynmously termed “pigmented lesions.” However, not all melanocytic proliferations are pigmented, and not all pigmented lesions are primarily melanocytic proliferations. Benign lesions composed of epidermal melanocytes include freckles, solar lentigines (“actinic” or formerly senile lentigo), the melanotic macules of Albright syndrome, and Becker melanosis. The cafe-au-lait patches of neurofibromatosis have been described elsewhere. Benign lesions derived from dermal melanocytes include the Mongolian spot, the nevi of Ota and of Ito, and the blue nevus. Benign tumors of nevus cells are called melanocytic nevi. They can be divided into junctional nevi including lentigo simplex, compound nevi, and intradermal nevi. There are special variants of melanocytic nevi, the more important of which include the Spitz nevus, the pigmented spindle cell nevus, the congenital melanocytic nevus, and the dysplastic nevus.
Dermal Melanocytoses and Hamartomas
It is important to distinguish dermal melanoses (implying only increase in dermal melanin pigment) caused by the presence of melanocytes in the dermis (melanocytoses) from those produced by the presence of melanin within non-melanocytic cells (e.g., macrophages) within the dermis. Clinically, the two different processes may have very similar appearances (7). In this section we consider the former condition. Dermal melanosis associated with metastatic melanoma is discussed in a later section of this chapter (p. 933), while inflammatory disorders associated with pigmentary alterations are discussed in Chapter 27.
Mongolian Spot
Clinical Summary. The typical Mongolian spot occurs in the sacrococcygeal region of an infant as a uniformly blue discoloration resembling a bruise. It consists of a noninfiltrated, round or ovoid, rather ill-defined patch of varying size. It is found more frequently in infants of Asian and African ethnicity. It is present at birth and usually disappears spontaneously within 3 to 4 years (8).
Occasionally, Mongolian spots occur outside the lumbosacral region as aberrant Mongolian spots, such as on the middle or upper part of the back; they may then be multiple and bilateral and persist. Extensive and persistent Mongolian spots are commonly seen in patients with bilateral nevus of Ota (9).
Histopathology. In the Mongolian spot, the dermis shows in its lower half or two-thirds greatly elongated, slender, often slightly wavy dendritic cells containing melanin granules that are distributed within the dendrites, rendering them visible. These cells are present in a low concentration and lie widely scattered between the collagen bundles, and like the collagen bundles, they generally lie parallel to the skin surface. No melanophages are seen.
Principles of Management. Education and reassurance are appropriate.
Nevi of Ota, Ito, and Hori; Dermal Melanocyte Hamartoma
Clinical Summary. Nevi of Ota and Ito and dermal melanocyte hamartoma are types of dermal melanocytosis that differ from the Mongolian spot by usually having a speckled rather than a uniform blue appearance and by showing a greater concentration of dermal melanocytes, with location in the upper rather than in the lower portion of the dermis (9,10).
The nevus of Ota is most often a unilateral discoloration of the face composed of blue and brown, partially confluent macules. The periorbital region, temple, forehead, malar area, and nose are usually involved. Because of this usual distribution, Ota called the lesion “nevus fuscocaeruleus ophthalmomaxillaris.” There is frequently also a patchy blue discoloration of the sclera of the ipsilateral eye and occasionally also a similar discoloration of the conjunctiva, cornea, and retina. In some instances, the oral and the nasal mucosa are similarly affected. In about 10% of the cases, the lesions of the nevus of Ota are bilateral rather than unilateral. They may be present at birth; they may also appear during the first year of life or during adolescence but only rarely in childhood. They have a tendency toward gradual extension. Malignant change in the cutaneous lesions of a nevus of Ota is extremely rare (9).
In the nevus of Ota, the involved areas of the skin show a brown to slate-blue uniform or mottled discoloration, usually without any induration. Occasionally, however, some areas are slightly raised. In some patients, discrete nodules varying in size from a few millimeters to a few centimeters and having the appearance of blue nevi are found within the areas of discoloration. Persistent Mongolian spots are quite common in association with the nevus of Ota. Extensive Mongolian spots are typically found in bilateral cases of nevus of Ota (9).
The nevus of Ito differs from the nevus of Ota by its location in the supraclavicular, scapular, and deltoid regions. It may occur alone or in association with an ipsilateral or bilateral nevus of Ota (11). Like the nevus of Ota, it has a mottled, macular appearance.
Nevus of Hori is a form of acquired dermal melanocytosis. It is also known as “acquired bilateral nevus of Ota-like macules” and “acquired symmetrical dermal melanocytosis” (12–14). The patients present with speckled blue–brown or gray macules that are located bilaterally in the malar region. The vast majority of the reported cases have been seen in Asian women. Histology reveals dendritic pigmented melanocytes in the mid and upper dermis. The dermal pigment stains positively with Fontana-Masson staining, and the melanin-containing cells are DOPA-positive. Electron microscopy has revealed a predominance of stage IV melanosomes and melanocyte dendrites encircling elastic fibers.
In the dermal melanocyte hamartoma, there may be a single, very extensive area of gray–blue pigmentation present from the time of birth. Histologic and ultrastructural examinations reveal numerous dermal melanocytes (15). The involvement may be nearly generalized (16). In other instances, there are several coalescing blue macules that have gradually extended within a circumscribed area from the time of childhood (17).
Histopathology. The noninfiltrated areas of the nevus of Ota, as well as the nevus of Ito and the dermal melanocyte hamartoma, show, like the Mongolian spot, elongated, dendritic melanized melanocytes scattered among the collagen bundles. However, in these three forms of dermal melanocytosis, the melanocytes generally are more numerous and more superficially located than in the Mongolian spot. Although most of the dendritic melanocytes lie in the upper third of the reticular dermis, melanocytes may also occur in the papillary layer, and may extend as far down as the subcutaneous tissue. Melanophages are seen in only few lesions (15,16). A histologic classification of Ota nevus has been proposed according to the distribution of the dermal melanocytes from superficial to deep. This correlates with the color and location of the nevus, and probably with response to therapy (18,19).
Slightly raised and infiltrated areas show a larger number of elongated, dendritic melanocytes than do noninfiltrated areas, thus approaching the histologic picture of a blue nevus, and nodular areas are indistinguishable histologically from a blue nevus (20).
Malignant changes in lesions of nevus of Ota have been reported in a handful of cases (21). The histologic appearance of the tumors may be that of a malignant or cellular blue nevus (CBN) (22). In a few instances, a primary melanoma of the choroid, iris, orbit, or brain has developed in patients with a nevus of Ota involving an eye (23,24). Copy number variations studied by comparative genomic hybridization have been described in one such case of orbital melanoma with histology ranging in different areas from that of nevus of Ota, to blue nevus, CBN, and melanoma. A benign or low-grade lesion termed a melanocytoma of the meninges may also occur (25).
In a study of 17 cases of nevus of Ota, and 46 cases of uveal melanoma, activating mutations in the cell surface signaling G protein GNAQ were found in 6% and 46%, respectively, providing a genetic basis for the implication of nevus of Ota as a low-level risk factor and a rare potential precursor of uveal melanoma (26).
Histogenesis of the Dermal Melanocytoses. The blue color of the dermal melanocytoses depends on the phenomenon whereby light passing through the skin is scattered as it strikes dark particles, such as melanin. Owing to this scattering phenomenon termed the Tyndall effect, the colors of light that have a longer wavelength, such as red, orange, and yellow, tend to be less scattered and therefore continue to travel in a forward direction, but the colors of shorter wavelength, such as blue, indigo, and violet, are scattered to the side and backward to the skin surface. This phenomenon is also responsible for the distinctive color of blue nevi, and of blood in veins.
The Mongolian spot is thought to be the result of the delayed disappearance of dermal melanocytes during embryogenesis. On electron microscopy, the dermal melanocytes are seen to contain numerous fully melanized melanosomes. Only a few melanocytes show premelanosomes as evidence of ongoing melanogenesis (8).
Because the concentration of melanocytes in the nevi of Ota and Ito and in the dermal melanocyte hamartoma is greater than in the Mongolian spot, it has been suggested that these lesions are nevoid or hamartomatous, rather than reactive or neoplastic. Although the lesional cells are considered to be melanocytes, the DOPA reaction may be negative, likely due to all melanogenic enzymes having been consumed in heavily pigmented melanocytes (15).
Principles of Management. Complete excision of the dermal melanocytoses is generally not possible. Consideration of periodic follow-up may be appropriate, especially for those lesions that involve the eye or the central nervous system.
Blue Nevi
Blue nevi are benign localized pigmented lesions that generally occur on the skin, although, occasionally, they may be observed in mucous membranes (27). On the skin, three types of benign blue nevi are recognized: the common blue nevus, the CBN, and the combined nevus. In addition, there are malignant blue nevi, discussed in a later section.
Histologically, the common feature of blue nevi is the presence of pigmented spindle and dendritic melanocytes in a focal area of the reticular dermis, associated (unlike the dermal melanocytoses) with alterations in the dermal collagen architecture. This deeply situated pigment differs from the pigment in acquired nevi that is typically superficial only, and accounts for the blue color of these lesions, due to the light-scattering Tyndall effect.
Common Blue Nevus
Clinical Summary. The common blue nevus occurs as a small, well-circumscribed, dome-shaped nodule of slate-blue or blue–black color (Fig. 28-1A). The lesion rarely exceeds 1 cm in diameter. Common blue nevi are frequently found on or near the dorsa of the hands and feet, as well as on the scalp. Usually, there is only one lesion, but there may be several. A rare manifestation is the plaque-type of blue nevus, which shows within a circumscribed area numerous macules and papules. This type of lesion may be present at birth or may become clinically apparent later in life (28). Malignant degeneration has not been reported in the common blue nevus.
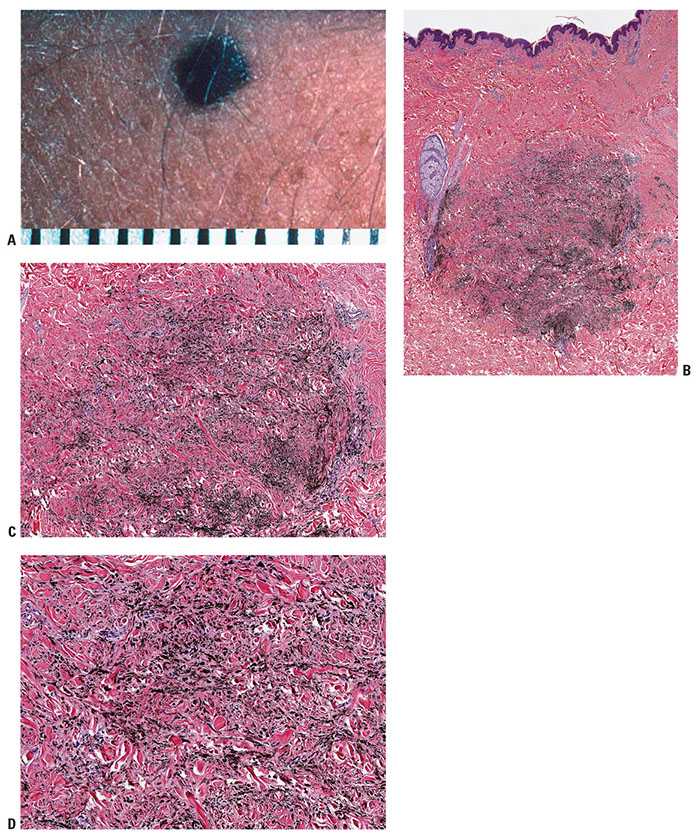
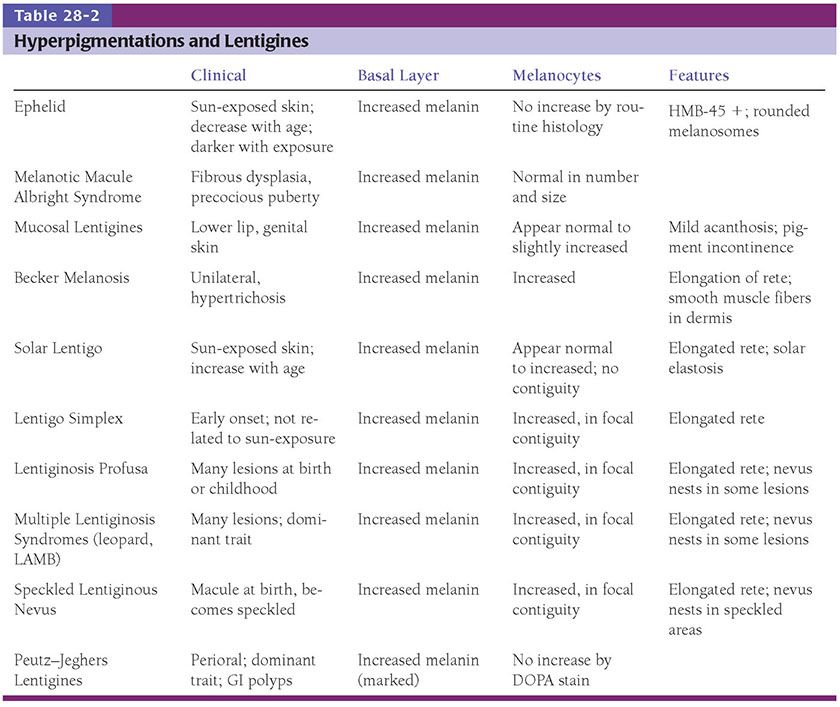
Figure 28-1 A: Blue nevus. A relatively small, well-circumscribed, slightly elevated blue–black pigmented lesion. B: Another somewhat more deep-seated lesion. Spindle-shaped or dendritic melanocytes are placed among reticular dermis collagen bundles, which are often slightly thickened. C: Unlike the cells of most common or congenital nevi that involve the reticular dermis, the cells of blue nevi are usually heavily pigmented, with coarsely divided melanin granules. D: Especially at the periphery of the lesion, the cells are arranged as single cells placed among collagen bundles, rather than sheets or fascicles.
Histopathology. In the common blue nevus, the melanocytes have a similar appearance to those in the Mongolian spot and in the nevus of Ota, but they are typically larger and their density is much greater. Greatly elongated, slender, often slightly wavy melanocytes with long, occasionally branching dendritic processes lie grouped in irregular bundles in the dermis (Fig. 28-1B–D). The bundles of cells may extend into the subcutaneous tissue or lie close to the epidermis. However, the epidermis is normal, except in the combined nevus (see p. 860). The greatly elongated melanocytes lie predominantly with their long axis parallel to the epidermis. Most of them are filled with numerous fine granules of melanin, often so completely that their nuclei cannot be visualized. The melanin granules also characteristically fill the long, often wavy, occasionally branching dendritic processes. Wavy fiber bundles similar to nerves may be present, indicative of Schwannian differentiation (29). Occasionally, lesional cells are seen in the perineurium of authentic nerves, a finding that is not indicative of malignancy. Melanophages may be seen near the bundles of melanocytes, but are usually not numerous or dense. The melanophages differ from the melanocytes by being more round to ovoid, by showing no dendritic processes, and by containing larger granules. The melanocytes of blue nevi are positive for S100 and HMB-45 (30) and for Melan-A (31). The number of fibroblasts and the amount of collagen are generally increased in blue nevi, resulting in disruption of the normal architecture of the connective tissue. In some lesions, the collagen is unusually prominent, and these have been termed desmoplastic blue nevi (or cellular blue nevi) (31,32). Despite their name, the color of blue nevi varies, with a variety of colors including blue, hypochromic, black, and brown variants, correlating to some extent with the depth and density of the lesional cells, and their melanization (33).
If melanocytes are relatively sparsely distributed and minimally pigmented, these lesions may be mistaken for a fibrohistiocytic lesion such as a dermatofibroma. A few blue nevi are hypopigmented or even amelanotic (34,35), requiring immunohistochemistry for their confirmation, with HMB-45-positivity representing a most helpful adjunct. On occasion, and particularly in small biopsies, the differential of desmoplastic melanoma versus amelanotic blue nevus may be raised, and again, HMB-45 positivity in the latter, but usually not in the former, is of assistance (36). In some cases, S100 and HMB45 staining may be weak or negative (34) and the use of other markers may then be considered (Immunohistochemistry, p. 919). Although blue nevi are usually strictly dermal, rare “compound blue nevi” have been described in which there are pigmented dendritic melanocytes in the epidermis near the dermal–epidermal junction (37). Junctional melanocytes may also be seen in the setting of “combined nevi” (see below). Recurrences of benign blue nevi after excision have been described; although this phenomenon is suggestive of malignancy and the recurrences tend to be more cellular than the original lesions, the behavior may be benign in the absence of compelling indicators of malignancy, such as necrosis, high-grade atypia, and mitoses (38).
Principles of Management. Although not generally considered mandatory, consideration of complete excision of blue nevi is appropriate, especially if a lesion has been partially sampled or was clinically dynamic or atypical, or if there are histologically “cellular” areas, or mitotic activity. In one study, persistence and recurrence of blue nevi was discussed, demonstrating that blue nevi of all histologic types and combinations are capable of persistence with clinical recurrence (38). The persistence usually is histologically similar to the original, but in some cases is more “cellular” and/or atypical. Limited follow-up of these cases has not demonstrated frankly malignant behavior. Clinical recurrence may also be associated with malignancy of a blue nevus-like lesion, but this study demonstrates that tumor progression to malignancy is not necessarily the case. In the absence of necrosis en masse, marked cytologic atypia, and frequent mitotic figures, recurrence of a blue nevus or a CBN is likely to be a benign phenomenon (38). However, we would recommend complete excision and follow-up for such recurrent lesions.
Cellular Blue Nevus
Clinical Summary. The CBN consists of a blue nodule that is usually larger than the common blue nevus. It generally measures 1 to 3 cm in diameter, but it may be larger. It shows either a smooth or an irregular surface. About half of all cellular blue nevi have been located over the buttocks or in the sacrococcygeal region (39–41). Although it is rare, malignant degeneration of cellular blue nevi can occur (40) (p. 928).
Histopathology. The profile of a CBN is often distinctive at scanning magnification, with a bulky, heavily pigmented cellular tumor often spanning the reticular dermis, and not associated with an overlying in situ melanoma (Fig. 28-2A–E). In lesions that enter the subcutis there is often a cellular nodule at the base, connected to the overlying tumor in a “dumbbell” pattern. Areas of deeply pigmented dendritic melanocytes, as seen also in the common type of blue nevus, are admixed with cellular islands composed of closely aggregated, rather large spindle-shaped or more epithelioid cells with ovoid nuclei and abundant pale cytoplasm often containing little or no melanin. Melanophages with abundant melanin may be present between the islands. Although pigment is usually prominent, amelanotic CBN have been described (36). Four histologic subtypes have been recognized: mixed biphasic, alveolar, fascicular, or neuronevoid (also known as the monophasic spindle cell–type), and atypical varieties (40). In the common mixed-biphasic type, there are clusters of epithelioid cells with somewhat clear cytoplasm, between which there are fascicles of spindle cells (Fig. 28-2E). Pigment is usually more prominent in the latter. The alveolar type is distinctive, characterized by rounded nests of clear spindle cells embedded in a matrix of dendritic and spindle-shaped, more heavily pigmented melanocytes similar to those of the mixed-biphasic pattern. The monophasic spindle cell–type is somewhat more problematic and may overlap with pigmented epithelioid melanocytomas (see p. 925) spindle cell tumorigenic melanomas (p. 917) and with malignant blue nevi (p. 928). The absence of an overlying in situ component may help to rule out the former. Attributes that may suggest malignancy in a CBN are discussed in the section on malignant blue nevi (p. 928). They include frequent mitoses, high-grade cytologic atypia, and spontaneous tumor necrosis or ulceration.
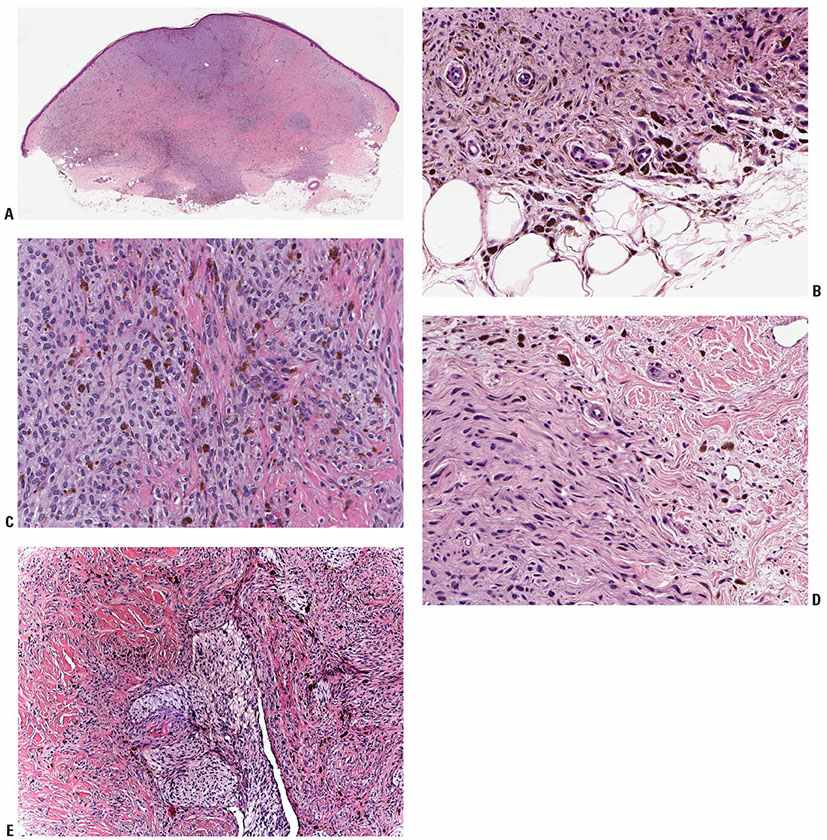
Figure 28-2 A: Cellular blue nevus. Usually broader at its surface than its base, the cellular blue nevus spans the reticular dermis, usually involving the superficial panniculus. Often, there is a region of increased cellularity that may form a bulbous expansion at the base. B: Spindle cells usually predominate in cellular blue nevi. They lie in contiguity with one another, unlike the cells of common blue nevi, most of which are separated from one another by collagen bundles. The lesional cells infiltrate into the panniculus at the tumor base. C: In the more “cellular” areas there are sheets and nests of cuboidal cells with pale cytoplasm, often separated by spindle cells. Mitoses are very rare in most examples. D: Changes at the periphery may be indistinguishable from those of a common blue nevus, although often with minimal pigment as here. E: Another lesion showing a characteristic “mixed-biphasic” pattern is a distinctive feature of many cellular blue nevi, with ovoid islands of polygonal cells with somewhat clear cytoplasm alternating with spindle cells, the latter often pigmented.
Lesions termed “atypical” cellular blue nevi have become recognized as a variant of cellular blue nevi, characterized by unusual features including architectural atypia (infiltrative margin and/or asymmetry) and/or cytologic atypia (hypercellularity, nuclear pleomorphism, hyperchromasia, occasional mitotic figures, and/or subtle necrosis) (39,40,42). Although most of these lesions have a benign course, a few cellular blue nevi (not necessarily all “atypical”) have been locally aggressive (43,44) or have metastasized at least to regional lymph nodes (45,46), and a guarded prognosis is appropriate in the presence of more than a few mitoses (see below, “Melanocytic Tumors of Uncertain Malignant Potential”, p. 930). The absence or scarcity of mitotic figures and the absence of areas of necrosis or of high-grade atypia are evidence against a diagnosis of malignant blue nevus, and the presence of areas of dendritic blue nevus-type cells elsewhere in the tumor as well as the lack of a characteristic intraepidermal (in situ melanoma) component argue against a diagnosis of melanoma. These criteria are somewhat ill-defined and a consensus diagnosis is often difficult to achieve (47).
In several instances in which cellular blue nevi were excised with regional lymph nodes under the mistaken diagnosis of melanoma, or because of an “atypical” or “uncertain” diagnosis, moderately or severely atypical cells of cellular nevus have been found in the regional lymph nodes, often in the marginal sinuses or in the capsule (48), but sometimes more massively involving the node (46). It is sometimes assumed that these cells do not represent true metastases but were passively transported to the lymph nodes and lodged there as inert deposits. However, some examples of this phenomenon in our experience have shown high-grade uniform atypia, necrosis, and fairly numerous mitoses, apparently indicative of an active neoplasm. Some of these lesions may best be interpreted as “Metastatic Tumors of Uncertain Malignant Potential” (p. 930).
Genomic aspects of blue nevi and related lesions have been increasingly documented. Somatic mutations of the G protein signaling molecule GNAQ were found in 83% of 29 blue nevi, indicative of an alternative pathway to MAP kinase activation in these lesions, differing from the BRAF or NRAS activation that is more usual in commonly acquired and many congenital pattern nevi (26). A FISH study of four chromosomal markers showed no changes meeting criteria for melanoma in 12 unambiguous cellular blue nevi (49). In a study using comparative genomic hybridization, 10 cellular blue nevi (lacking equivocal histologic features) showed no chromosomal abnormalities (50).
Despite these negative genomic findings, in rare cases, a CBN is seen adjacent to an unequivocally malignant melanocytic proliferation. This phenomenon has been termed “malignant blue nevus” or “melanoma arising in a cellular blue nevus,” and indicates that these lesions have some low but finite potential for progression and malignant transformation (see p. 928).
Histogenesis of Blue Nevi. There is general agreement that the cells of the common blue nevus are melanocytes, which may show evidence of Schwannian differentiation. This occasional resemblance to neural tumors had led in the past to suggestions of neural origin for blue nevi (51). However, the lesional cells of blue nevi and their variants react positively with antibodies to the S100, HMB-45, and Melan A/MART-1antigens, the latter two in this context being quite specific for melanocytic differentiation, and also serving to help differentiate these lesions from desmoplastic melanomas that are typically negative for these markers (31,52). Also, melanosomes are present by electron microscopy (EM 48), and the electron microscopic DOPA reaction indicates that the spindle-shaped cells of the CBN have melanogenic potential (53).
Principles of Management. Lesions in this general category at a minimum should be excised completely, not only to ensure that the lesion has been completely examined using histologic techniques, but also to minimize any chance of persistence, recurrence, and progression to a more significant lesion (47). With regard to local persistence, it is of note that such lesions recurring in scar tissue may produce additional diagnostic complexities, including potentially increased risk for melanoma overdiagnosis.
Combined Nevus
Clinical Summary. The term combined nevus is applied to the association of a blue nevus or a deep-penetrating nevus with an overlying melanocytic nevus, or to other combinations of benign nevi (54). Clinically, combined nevi often present with a focal area of deep pigmentation.
Histopathology. In a combined nevus, one component is often a congenital pattern nevus in which pigmented spindle cells form a focal collection of fascicles among nests of ordinary nevus cells. The term “clonal nevus” has been applied to some of these lesions. The fascicles tend to be organized along the lines of a blue nevus, or, perhaps most frequently, a deep-penetrating nevus (55). The other component may be an overlying and/or adjacent nevus of the junctional, compound, intradermal or, rarely, Spitz nevus types (54). Some of the latter are examples of lesions in which there is loss of the tumor suppressor gene BAP1 in the spitzoid component, with preservation of expression in the background nevus (56). Often the dermal component has “congenital pattern features” (involvement of the reticular dermis and skin appendages, see p. 888). In a study of 220 cases, a “common nevus” component (with or without dysplastic or congenital pattern features) was present in 178 cases, a “blue nevus” component (dendritic, cellular blue, or deep-penetrating nevus) in 147 cases, and a “Spitz nevus” component in 37 cases. The most common combination was a commonly acquired compound nevus type associated with a deep-penetrating nevus, which occurred in 57 cases (31.3%). A Spitz nevus was associated with a blue nevus in six cases (57).
Such lesions may simulate melanoma clinically, because of the appearance of a very darkly pigmented spot within a background nevus. Histologically, the pigmented spindle cells of the blue nevus component may give rise to suspicion based on apparent asymmetry of pigment distribution, but mitoses and high-grade atypia are absent, and the pigmented cells often blend with the background nevus rather than displacing or destroying it. Further, it is extremely unusual for a melanoma to arise in the dermal component of a nevus in the absence of a characteristic intraepidermal component.
Principles of Management. Although not generally considered mandatory, consideration of complete excision of combined nevi is appropriate in our opinion, especially if a lesion has been partially sampled or was clinically dynamic or atypical, or if there are histologically “cellular” areas, or mitotic activity.
Freckles and Hyperpigmentations
By definition, a freckle (ephelid) is a small flat tan or brown lesion that histologically shows increased pigment in keratinocytes, but no increase in the number of melanocytes. Hyperpigmentations may be described as larger macular pigmented lesions that show increased melanin pigment in keratinocytes without melanocytic proliferation. Lentigines are macular hyperpigmentations that differ from freckles and hyperpigmentations in that the number of epidermal melanocytes is increased within the basal cell layer. However, in common parlance, the term “freckle” is often used to refer both to ephelids and to solar lentigines, as well as other forms of macular hyperpigmentations. Ephelids appear early in childhood and are associated with fair skin type and red hair. Solar lentigines appear with age and are a sign of photodamage. Both lesions are strong risk indicators for melanoma and nonmelanoma skin cancer. Freckles are strongly influenced by the melanocortin-1-receptor (MC1R) gene, which has been termed “the freckle gene” and is also associated with fair skin, red hair, and melanoma and nonmelanoma skin cancer. In a large case-control study, carriers of MC1R gene variants had a markedly increased risk of developing ephelides, whereas the risk of developing severe solar lentigines was moderately increased, suggesting that MC1R is a major gene controlling susceptibility to the development of these forms of macular hyperpigmentations (58).
Principles of Management of Freckles. Freckles generally do not require active therapy. As discussed above, they are markers of skin at risk for development of melanoma, and as such they may play a part in a decision to offer skin surveillance. In some circumstances, such as Albright syndrome, freckles may have significance in diagnosis of a systemic condition.
Simple Ephelid (Freckle)
Clinical Summary. Freckles, or ephelids, are small, red–brown macules scattered over skin exposed to the sun. Exposure to the sun deepens the pigmentation of freckles; in contrast to lentigo simplex, whose already deep pigment does not change. Ephelids, simple lentigines, and solar lentigines are difficult to distinguish from one another clinically, and are considered together in most clinical and epidemiologic studies. Taken together, these lesions constitute a significant risk factor for the development of melanoma (59).
Histopathology. Freckles show hyperpigmentation of the basal cell layer, but in contrast to lentigines, there is no elongation of the rete ridges and, by definition, no obvious increase in the concentration of melanocytes. However, in a quantitative study of lesions from children, melanocyte frequencies in freckles were significantly greater than in adjacent nonpigmented skin. Cellular atypia of melanocytes, and reactivity of melanocytes for HMB-45 were noticed in some freckles (60). It is likely that freckles represent a hyperplastic and hyperactive response of melanocytes to UV light.
Histogenesis. On electron microscopy, the melanocytes within freckles are found to be essentially similar to those of darker skinned persons. Melanocytes of the surrounding epidermis, by contrast, in freckle-prone subjects show constitutionally few and minimally melanized melanosomes, many of which are rounded rather than elongated (61). Such rounded melanosomes (so-called pheomelanosomes) are characteristic of the lightly pigmented skin of individuals with red hair and/or blue eyes and a cutaneous phenotype that is prone to freckles. As noted above, the tendency to develop freckles appears to be closely related to MC1R gene polymorphisms (58).
Melanotic Macules of Albright Syndrome
Clinical Summary. Albright syndrome usually is characterized by unilateral polyostotic fibrous dysplasia, precocious puberty in females, and melanotic patches. The patches generally are large in size and few in number, are located on only one side of the midline, often on the same side as the bone lesions, and have a jagged, irregular border, like the “coast of Maine,” in contrast to the smooth “coast of California” type of border of the cafe-au-lait patches of neurofibromatosis.
Histopathology. Except for hyperpigmentation of the basal layer, there is no abnormality, and both the number and the size of the melanocytes are normal (62).
Differential Diagnosis. The melanotic macules of Albright syndrome only rarely show the “giant” melanin granules (macromelanosomes) that are commonly seen in some of the melanocytes and keratinocytes within the cafe-au-lait patches of neurofibromatosis. Histologically, the melanotic macules may be indistinguishable from ephelides without correlative clinical information.
Mucosal Melanotic Macules (Mucosal Lentigines)
Clinical Summary. These benign lesions present as a pigmented patch on a mucous membrane. Common locations include vermilion border of the lower lip, the oral cavity, the vulva and, less often, the penis. These lesions may simulate melanoma clinically but histologically there is no contiguous melanocytic proliferation and no significant atypia. Because there may be a slight increase in the number of melanocytes, though there is no nest formation, the term “genital lentiginosis” has been proposed for those lesions affecting genital paramucosae (63). The lesions may be synonymously referred to as “mucosal lentigo” or “mucosal melanotic macule.”
In the common location on the vulva (“vulvar lentigo”), this process may appear quite alarming clinically, presenting as a broad, irregular, and asymmetric patch of brown to blue–black hyperpigmentation, often meeting the “ABCD” criteria for melanoma discussed below (64). Similar lesions may also be seen on penile skin (63). The lesions may be multicentric with alternating areas of normal and pigmented mucosa resembling areas of partial regression of a melanoma. The lesions are entirely macular, which would be unusual in an invasive melanoma.
The so-called “labial lentigo” (also known as “labial melanotic macule” or “labial melanosis”), a hyperpigmented macule of the lip, is quite similar to the lesions of genital skin. It is rarely biopsied because the clinical appearances are characteristic and do not suggest malignancy. These lesions are uniformly pigmented light to dark brown, usually completely macular, and usually less than about 6 mm in diameter. These macules are biologically indolent (65).
Histopathology. Upon initial inspection, a biopsy specimen may appear normal. The findings include mild acanthosis without elongation of rete ridges, and hyperpigmentation of basal keratinocytes, often best recognized in comparison with surrounding epithelium, in association with scattered melanophages in the dermis. Although melanocytes may be normal in number, in most instances, they are slightly increased (63,66). Because of this slight increase in number, the lesions are termed lentigines (as opposed to ephelids) by strict histologic criteria (63). In contrast to true melanocytic neoplasms (nevi or melanomas), the cell bodies of the lesional melanocytes are separated by those of keratinocytes, that is, there is no contiguous proliferation of melanocytes. Occasionally, especially in the penile and vulvar lesions, there are prominent dendrites of melanocytes ramifying among the hyperpigmented keratinocytes. There may be associated mild keratinocytic hyperplasia, and scattered melanophages in the papillary dermis, resulting from pigmentary incontinence, may account for the blue–black color and the pigmentary variegation that may simulate melanoma clinically.
Differential Diagnosis. The histologic distinction from radial growth phase (RGP) melanoma is easy because of the absence of neoplastic (contiguous) melanocytic proliferation and cytologic atypia of melanocytes (67).
Histogenesis. The process appears likely to be one of reactive hyperplasia with some features of post-inflammatory hyperpigmentation, rather than a neoplasm (68). The phenomenon is benign with associated lesional growth stabilization over time.
Principles of Management. These lesions may need to be biopsied to rule out melanoma. If there is melanocytic atypia, complete excision and follow-up should be considered.
Becker Melanosis
Clinical Summary. Becker melanosis, also called Becker pigmented hairy nevus, occurs most commonly as a large, unilateral patch showing hyperpigmentation and hypertrichosis on the shoulder, back, or chest of an adult male (69). Usually, the patch is sharply but irregularly demarcated. Occasionally, however, the lesion presents as coalescing macules instead of a solitary patch. The lesion commonly appears during the second decade of life. In some instances, Becker melanosis affects areas other than the shoulder and chest. Also, it may be multiple and bilateral, and may occasionally be found in women.
In one report, nine cases of melanoma in association with Becker nevus were described (70). Five of these were on the same body site as the nevus. It remains to be determined whether these reports represent a greater incidence than chance would suggest.
The hairiness appears after the pigmentation, and, quite frequently, no hypertrichosis is seen. It is therefore possible that cases described as progressive cribriform and zosteriform hyperpigmentation represent a variant of Becker melanosis without hypertrichosis (71).
Of interest is the association of a pilar smooth muscle hamartoma with Becker melanosis (p. 862). In such cases, the area of Becker melanosis may show slight perifollicular papular elevations or slight induration (72).
Histopathology. The epidermis shows slight acanthosis and irregular elongation and flattening with a tendency for fusion of the rete ridges. There is hyperpigmentation of the basal layer, and melanophages are seen in the upper dermis. The number of melanocytes is increased within the basal cell layer, as has been demonstrated using image analysis and staining with melanocyte markers including S100 and MART-1. In the same study, androgen receptor expression was increased in the epidermis (73), suggesting a possible pathogenetic mechanism. The pilar structures may appear normal or increased in number.
An increase in smooth muscle fibers exists in nearly all cases, although it may be slight. In cases with an associated smooth muscle hamartoma, irregularly arranged, thick bundles of smooth muscle are present in the dermis (72). The term Becker nevus syndrome has been proposed for a phenotype characterized by the presence of a particular type of organoid epithelial nevus showing hyperpigmentation, increased hairiness, and hamartomatous augmentation of smooth muscle fibers (smooth muscle hamartoma), and other developmental defects such as ipsilateral hypoplasia of breast and skeletal anomalies including scoliosis, spina bifida occulta, or ipsilateral hypoplasia of a limb (74).
The few melanomas that have been described in association with Becker nevus have been of the superficial spreading type, originating in the epidermis (70).
Principles of Management. These lesions may be biopsied if thought necessary to rule out melanoma.
Lentigines
Lentigines are macular hyperpigmentations in which the number of epidermal melanocytes is increased and there are no nests of melanocytes as are present, by definition, in nevi. The term “lentigo” is derived from the Latin “lenz” meaning lens or lentil (75). Thus, the term in its original usage is clinical, referring to a small ovoid or lens-shaped pigmented spot. The term has come to be applied to larger pigmented lesions, especially those that recapitulate to a greater or lesser extent the histologic features of a lentigo simplex: basal proliferation of melanocytes arranged as single cells rather than in nests, typically but not always associated with elongation of the rete ridges. This pattern of melanocytic proliferation is termed “lentiginous.” Lentiginous melanocytic proliferation is seen in the macules of solar lentigo and lentigo simplex, and in the macular or plaque components of lentiginous junctional and compound nevi, of lentiginous dysplastic nevi, and of lentiginous melanoma, including lentigo maligna, acral, and mucosal-lentiginous types.
Solar Lentigo (Actinic Lentigo)
Clinical Summary. Solar lentigines commonly occur as multiple lesions in areas exposed to the sun, such as the face and extensor surfaces of the forearms, but most commonly on the dorsa of the hands. The lesions increase in number with age, in contrast to nevi that decline in number (76). Therefore, they have been referred to as senile lentigines. However, sun exposure, rather than age, is the eliciting factor (77). Thus, the lesions do not occur on sun-protected skin, even in the elderly. Solar lentigines are commonly seen in sun-exposed Caucasoids. They are not indurated, possess a uniform dark brown color, and have an irregular outline. They vary in diameter from minute to more than 1 cm, and may coalesce. Solar lentigines, like ephelides, are risk markers for the development of melanoma (78), and are commonly numerous in the skin around melanomas, as seen in melanoma reexcision specimens. Lesions termed “sunburn freckles” by some clinicians overlap clinically and histologically with actinic lentigines. They are blotchy macular areas of tan hyperpigmentation, often of the order of 1 cm in diameter, which often appear on the shoulders or other sun-exposed areas of a young person after a severe sunburn (79). Other potentially related lesions are intensely dark, perfectly macular reticulated lesions that have been called “reticulated lentigo” (80) or “ink-spot lentigo” (81).
Solar lentigines differ from ephelides in that they are more prevalent, increase in prevalence and number with higher age (ephelides tend to decline in number), and are most prevalent on the trunk. They occur more frequently in males than in females, unlike ephelides, which are more evenly distributed and are not related to sun exposure (82,83).
Solar lentigines and relatively flat seborrheic keratoses may resemble each other in clinical appearance, and both are commonly referred to as “liver spots” or “age spots.” Seborrheic keratoses in general show more hyperkeratosis clinically. In contrast, lentigo maligna differs from solar lentigo in clinical appearance by its irregular distribution of pigment, often in a finely reticulated pattern, and by its greater asymmetry and border irregularity (p. 911).
A recent microarray analysis of solar lentigines demonstrated upregulation of genes related to inflammation, fatty acid metabolism, and melanocytes, and downregulation of cornified envelope-related genes; the authors suggest that solar lentigo may be induced by the mutagenic effect of repeated ultraviolet light exposures, leading to enhancement of melanin production along with decreased proliferation and differentiation of lesional keratinocytes on a background of chronic inflammation (84). It has been postulated that abnormal pigment retention in keratinocytes may be the primary disorder in solar lentigines (85).
Prolonged treatment with psoralen and ultraviolet light A (PUVA) can induce formation of pigmented macules (“PUVA lentigines”) in the irradiated areas. These are similar to solar lentigines but their color is darker and their pigment is more irregularly distributed (86).
Histopathology. A recent study from Japan described two patterns of solar lentigines on skin of the face, both associated with hyperpigmentation of basal keratinocytes and an increased number of single basal melanocytes. In one pattern, there was a flattened epidermis with basal melanosis, and the other pattern there was epidermal hyperplasia with elongated rete ridges composed of deeply pigmented basaloid cells (87). In the latter or “budding” pattern, the rete ridges are subtly or more significantly elongated. They either appear club-shaped or are tortuous and show small, bud-like extensions. The elongated rete ridges are composed, especially in their lower portion, of deeply pigmented basaloid cells intermingled with melanocytes, which are arranged mostly as single cells. Epidermal maturation may appear subtly perturbed in some lesions. The melanocytes appear significantly increased in number in some cases, but only slightly or not at all increased in others (Fig. 28-3). They possess a heightened capacity for melanin production, as shown by the fact that, on staining with DOPA, they display more numerous as well as longer and thicker dendritic processes than the melanocytes of control skin (88). The upper dermis shows elastosis and often contains scattered melanophages and occasionally a mild, perivascular lymphoid infiltrate. In the “flattened epidermis” pattern, there was a significantly thinner epidermis, more severe solar elastosis and fewer Langerhans cells in the epidermis as compared with the budding group. In some lesions, the rete ridges are elongated to such an extent that strands of basaloid cells form anastomosing branches, resulting in a reticulated pattern closely resembling that seen in the reticulated pigmented type of seborrheic keratosis. However, unlike seborrheic keratoses, solar lentigines do not form horn cysts.
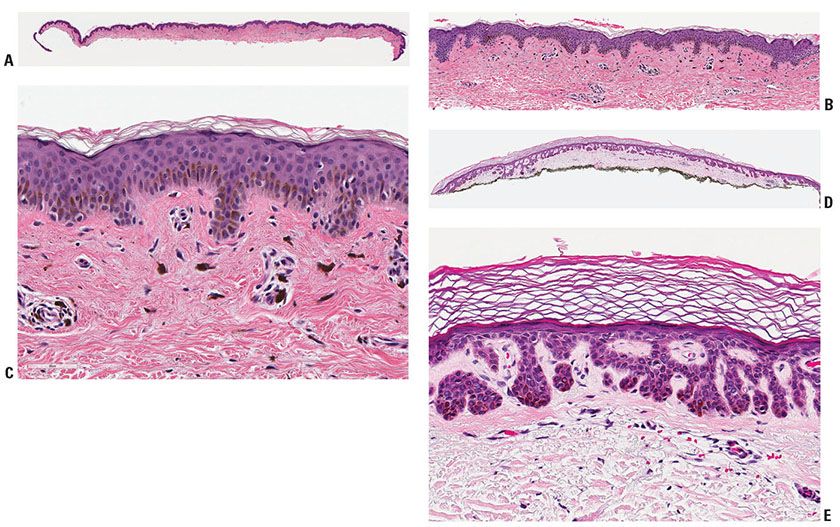
Figure 28-3 Actinic lentigo. A: Scanning magnification showing a localized area of elongated rete ridges in elastotic actinically damaged skin. B: Higher magnification shows basal hyperpigmentation and slight-to-moderate prominence of melanocytes, without contiguous proliferation. C: Sometimes there is slight atypia of randomly scattered melanocytes (mild random atypia). D: Another example shows more prominent rete ridge changes across a broad extent. E: Actinic lentigines are characterized by some combination of hyperpigmentation of basal keratinocytes, elongation of rete ridges, and prominence of melanocytes, the latter not especially marked in this example.
Solar elastosis is a marker for chronic solar damage (CSD), which is linked to the prevalence and distribution of solar lentigines and other sun-related conditions including malignant melanoma. A system for the scoring of the degree of solar elastosis has been developed and validated. The following scoring system was used: CSD 0: absence of elastotic fibers visible at 200× magnification; CSD 1: scattered elastotic fibers lying as individual units, not as bunches, between collagen bundles; CSD 2: densely scattered elastotic fibers distributed predominantly as bushels rather than individual units; CSD 3: amorphous deposits of blue–gray material with lost fiber texture (89). Solar elastosis is also discussed and illustrated in Chapter 12.
Solar lentigines differ histologically from ephelides by definition, in having an increased number of epidermal melanocytes. However, in some lesions, the proliferation may be demonstrable only by formal counting (90). In contrast to lentigo simplex, lentiginous nevi, and lentiginous melanomas, the melanocytic proliferation is not contiguous and is non-nested.
PUVA-induced pigmented macules represent solar lentigines on the basis of irregular elongation of their rete ridges. They show an increased number of large melanocytes that may appear slightly atypical (91).
Large cell acanthoma, which presents as a slightly scaly tan macule on photodamaged skin, is identified histologically by having epidermal keratinocytes with nuclei roughly twice the size of adjacent keratinocytes, but with minimal nuclear pleomorphism. There are clinical, histologic, and immunohistochemical overlapping characteristics with solar lentigo, suggesting that large cell acanthoma should be considered as a related condition (92). The lesions are distinguished from actinic keratoses by the lack of cytologic atypia (except for the nuclear enlargement) and the lack of parakeratosis.
In the reticulated or “ink-spot” lentigo, histologic evaluation, including electron microscopy and DOPA-incubated vertical sections, demonstrated lentiginous hyperplasia of the epidermis, marked hyperpigmentation of the basal layer with “skip” areas that involved the rete ridges, and a minimal increase in the number of melanocytes (81).
Histogenesis. By electron microscopy, the basal layer of keratinocytes contains increased melanosomes and melanosome complexes, and the melanosome complexes within keratinocytes appear larger than those found in uninvolved skin. Even in the upper layers of the epidermis, including the horny layer, numerous melanosomes are present largely in a dispersed state rather than as complexes (85).
Differential Diagnosis. In lentigo simplex (see below), the rete ridges are elongated, but in contrast, the lesional melanocytes are more obviously increased in number and focally lie in contiguity with one another around the tips and sides of the rete, but not between the rete. Lentigo maligna often shows flattening or absence of the rete ridges together with contiguous and continuous proliferation and uniform atypia of its melanocytes; like lentigo simplex, however, it may be associated with a dermal lymphocytic infiltrate. In actinic lentigo, the rete are elongated and the lesional melanocytes do not lie in contiguity with one another, even though they may be increased in number. There is minimal cytologic atypia, and no pagetoid spread of melanocytes above the basal layer. In contrast to a pigmented actinic keratosis, there is no keratinocytic atypia and usually no parakeratosis.
Principles of Management. Lentigines generally do not require active therapy. As discussed above, they are markers of skin at risk for development of melanoma, and as such they may play a part in a decision to offer skin surveillance. Occasional lesions exhibit clinical atypia sufficient to prompt biopsy to rule out melanoma.
Lentigo Simplex and Related Lesions
Clinical Summary. Simple lentigines are macular hyperpigmentations in which the number of epidermal melanocytes is increased. In solar lentigines, as described above, the cell bodies are separated from one another by those of keratinocytes, and the proliferation may be termed “noncontiguous.” The proliferation may be described as “contiguous” if the cell bodies at least focally touch one another, as in lentigo simplex (and also in lentiginous nevi and in lentiginous melanomas).
Lentigo simplex most frequently arises in childhood, but it may appear at any age (93). Usually in lentigo simplex, there are only a few scattered lesions without predilection to areas of sun exposure. They are small, symmetrical, and well-circumscribed macules that are evenly pigmented but vary individually from brown to black (Fig. 28-4A). They are not indurated and usually measure only a few millimeters in diameter. Clinically, a lentigo simplex is indistinguishable from a junctional nevus. Special forms of lentigo simplex are lentiginosis profusa, the multiple lentigines syndrome or leopard syndrome, and speckled lentiginous nevus, also referred to as nevus spilus.
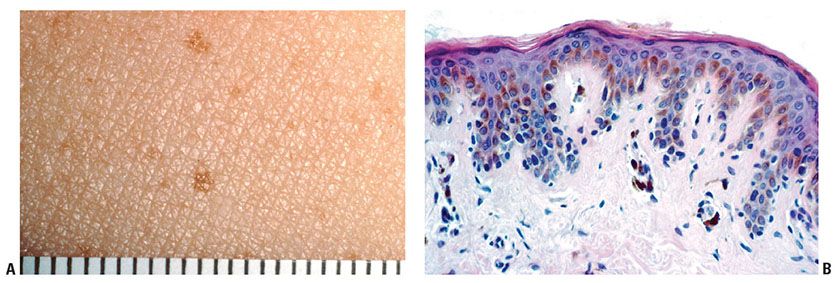
Figure 28-4 Lentigo simplex. A: Clinically, the lesions are small, usually less than 2 mm, fairly symmetrical and well-circumscribed. It is not possible to distinguish clinically among ephelides, simple lentigines, and lentiginous junctional nevi. (Clinical photograph by Peter Wilson.) B: Melanocytes are present in contiguity near the tips and sides of elongated rete ridges “lentiginous proliferation.” There is no “continuous” proliferation between the rete. The presence of at least a single nest would define a lentiginous junctional nevus (“jentigo”).
Lentiginosis profusa shows innumerable small, pigmented macules either from birth or starting in childhood or early adulthood without any other abnormalities. The mucous membranes are spared. There may be a family history (94,95). Agminated or segmental lentigines have been defined as a circumscribed group of small pigmented macules arranged in a small or large group, often in a segmental pattern, each macule consisting of a lentiginous intraepidermal proliferation of melanocytes (96,97). The speckled lentiginous nevus, or nevus spilus, consists of a light brown patch or band present from the time of birth that in childhood becomes dotted with small, dark brown macules (98).
The multiple lentigines syndrome, a dominant trait, is characterized by the presence of thousands of flat, dark brown macules on the skin but not on the mucous surfaces. The lentigines begin to appear in infancy and gradually increase in number. Although most macules vary from pinpoint dots to 5 mm in diameter, some dark spots are much larger, up to 5 cm in diameter. Features of this rare syndrome known also by the mnemonic leopard syndrome, in addition to the lentigines (L), may include electrocardiographic conduction defects (E), ocular hypertelorism (O), pulmonary stenosis (P), and abnormalities of the genitalia (A) consisting of gonadal or ovarian hypoplasia, retardation of growth (R), and neural deafness (D). Not all of these manifestations are present in every case. Cardiomyopathy may also be present and associated with significant mortality (99). Another syndrome associated with lentigines is known under the acronyms NAME or LAMB or myxoma syndrome (lentiginous nevi, atrial and/or mucocutaneous myxomas, myxoid neurofibromas, ephelides, blue nevi). It has been proposed that these mnemonics be dropped because the particular features encompassed within this syndrome are unclear, and the term “cutaneous lentiginosis with atrial myxomas” is an adequate description of this syndrome (100). The Carney complex, a familial multitumoral syndrome, comprises spotty skin pigmentation (lentigines and blue nevi), myxomas (heart, skin, and breast), endocrine “overactivity” usually manifested by endocrine tumors (adrenal cortex, pituitary, testis, and thyroid), schwannomas, and two unusual pigmented tumors, epithelioid blue nevus (skin), and psammomatous melanotic schwannoma (involving skin, viscera, or nerve tissue). Carney complex has been linked to chromosome 2p16 and the PRKAR1A gene at 17q22–24 (101).
The Peutz–Jeghers syndrome (PJS) shows dark brown macules clinically resembling lentigines in the perioral region. Similar macules are seen on the vermilion border and the oral mucosa and, often, the dorsa of the fingers. Although a few cases of this dominantly inherited disorder have shown only the pigmentary anomaly, there are usually multiple polyps in the gastrointestinal tract, mainly in the small intestine (102). Although early reports did not demonstrate a predisposition to cancer in patients with this syndrome, more recent studies have described an increased risk for both gastrointestinal and extra-gastrointestinal cancers. Women with the PJS have an extremely high risk for breast and gynecologic cancer. Recently, a PJS susceptibility gene, encoding the serine threonine kinase STK11 (also called LKB1), was identified in this syndrome (103). A complex of PJS-like pigmentation without polyposis, designated as isolated mucocutaneous melanotic pigmentation (IMMP), has recently been described, and also appears to be associated with increased risk of cancer in diverse organs (104).
Histopathology. Lentigines, in general, show a slight or moderate elongation of the rete ridges, an increase in the concentration of melanocytes in the basal layer, an increase in the amount of melanin in both the melanocytes and the basal keratinocytes, and the presence of melanophages in the upper dermis (93). The melanocytes in the epidermis lie in focal contiguity with one another near the tips and sides of the elongated rete, but the proliferation is not continuous between the rete (Fig. 28-4B). There are no nests by definition. In some instances, melanin is also seen in the upper layers of the epidermis, including the stratum corneum. A mild inflammatory infiltrate may be intermingled with the melanophages within the underlying papillary dermis. In lesions otherwise clinically characteristic of lentigo simplex, small nests of nevus cells are commonly seen at the epidermal–dermal junction, especially at the lowest pole of rete ridges. The lesions then combine features of a lentigo simplex and a junctional nevus, leading to their descriptive diagnosis as “jentigo” (105) or “lentiginous junctional nevus.” Because of the existence of these transitional forms, the lentigo simplex is regarded as a potential precursor of what may become a melanocytic nevus, and is discussed as such in a later section (p. 868).
In lentiginosis profusa and the multiple lentiginosis syndromes, as a rule, the lesions are “pure” lentigines without the formation of nevus cell nests. In larger macules, however, there may be junctional nevus cell nests, and there may even be nevus cell nests in the upper dermis (106).
In speckled lentiginous nevus, or nevus spilus, the light brown patch or band shows the histologic features of lentigo simplex. The speckled areas show junctional nests of nevus cells at the lowest pole of some of the rete ridges, diffuse lentiginous melanocytic proliferation, as well as dermal aggregates of nevus cells. Various types of nevi (e.g., junctional nevi, blue nevi, and Spitz nevi) may present in the same lesion over time, and histologic features of CMN may be present within the spots, suggesting that these lesions may be considered as variants of congenital nevi (98).
In the lesions of PJS, the basal cell layer shows marked hyperpigmentation. Although the number of melanocytes may appear to be slightly increased, no increase has been found in DOPA-stained sections (107). The intestinal polyps appear to be hamartomas, because glands are intermingled with smooth muscle bundles (103).
The presence of occasional giant melanin granules has been described in various forms of lentigines and lentiginous nevi, as well as in other conditions associated with hyperpigmentation, including the cafe-au-lait spots of neurofibromatosis and, less commonly, in cafe-au-lait spots without neurofibromatosis, and, on occasion, even in normal skin of healthy persons (108). Thus, they have no diagnostic specificity. Giant melanin granules vary in size from 1 to 6 μm. Because of their size and heavy melanization, the larger granules are readily recognized by light microscopy. Although seen largely within melanocytes, they also occur in keratinocytes and melanophages to which they have been transferred, although many are too large for conventional donation by affected melanocytes to keratinocytes. On electron microscopy, the giant melanin granules have been termed macromelanosomes, and are regarded as autolysosomes referred to as “melanin macroglobules” and represent lysosome-mediated accumulation of melanosomes to form massive rounded to ellipsoid melanized bodies (109).
Table 28-2 summarizes some of the salient clinical and histologic features observed in the various forms of hyperpigmentations and lentigines:
Principles of Management. Simple lentigines and related lesions generally do not require active therapy.
Melanocytic Nevi
Although the term “nevus” may refer to a variety of hamartomatous and/or neoplastic lesions in the skin, the unqualified term in common usage and in this chapter refers to a melanocytic nevus, which is generally considered to be a benign neoplastic proliferation of melanocytes, leading to a localized pigmented or nonpigmented lesion usually less than 5 mm in diameter.
Common Melanocytic Nevus
Clinical Summary. Nevi vary considerably in their clinical appearance. In addition to the pathologic variants, which will be discussed separately, five clinical types may be recognized: (a) flat lesions, (b) slightly elevated lesions often with a raised center and a flat periphery, (c) papillomatous lesions, (d) dome-shaped lesions, and (e) pedunculated lesions. The first three types are always pigmented; the latter two may or may not be pigmented. Any of the elevated lesions may be surrounded by a flat periphery, within which changes of melanocytic dysplasia may be seen histologically (“nevus with dysplasia”). Dome-shaped lesions often contain several coarse hairs. Although exceptions occur, one can predict to a certain degree from the clinical appearance of a nevus whether on histologic examination it will prove to be a junctional nevus (confined to the epidermis), a compound nevus (epidermal and dermal), or an intradermal nevus. Most small flat lesions represent either a lentigo simplex or a junctional nevus; flat lesions or lesions with a flat periphery 5 mm or more in diameter with irregular indefinite borders and pigment variegation are clinically dysplastic nevi, although if these changes are severe, melanoma may need to be ruled out. Most slightly elevated lesions and some papillomatous lesions represent compound nevi (especially if they are pigmented), and most papillomatous lesions and nearly all dome-shaped and pedunculated lesions that are not pigmented represent intradermal nevi.
Melanocytic nevi are only rarely present at birth (see “Congenital Melanocytic Nevus,” p. 868). Most nevi appear in adolescence and early adulthood. In this age period, they may occur episodically and, rarely, as widespread eruptive nevi (110–112). Occasionally, new nevi arise in midlife and rarely in later life. Except for occasional cosmetic significance, nevi are important only in relation to melanoma, for which they are risk markers, simulants, and potential precursors (78,93,113).
A general concept of clinical importance for melanocytic nevi is that, unlike melanoma that inexorably progresses over time, nevi enlarge to a point, stabilize, and then involute, becoming less frequent in the elderly than in younger age groups (76). This clinical attribute is directly related to the importance of heightened suspicion that is aroused when a previously stable nevus undergoes change in size or pigmentation.
Histopathology. Melanocytic nevi are defined and recognized by the presence of nevus cells, which, even though they are melanocytes, differ from ordinary melanocytes in three morphologic attributes: they are arranged at least partially in clusters or “nests”; they have a tendency to appear round rather than have a dendritic cell shape; and they have a propensity to retain pigment in their cytoplasm rather than to transfer it to neighboring keratinocytes (114). Nevus cells show considerable variation in their appearance, and often are not pigmented, so that they are often recognizable as nevus cells more by their arrangement in clusters or nests than by their cellular features. As the result of a characteristic shrinkage artifact, nevus cell nests often appear partially separated from their surrounding stroma, and in some nevi such as the spindle and epithelioid cell variant, from surrounding epithelium.
Although a histologic subdivision of nevi into junctional, compound, and intradermal nevi is generally accepted, it should be realized that these are transitional stages in the “life cycle” of nevi, from junctional to compound to intradermal, and finally to involuting lesions. The concept of progression from a lentigo simplex to a junctional and then a compound nevus has been challenged by the finding that BRAF mutations are more common in compound and dermal than in junctional nevi, and are not found in simple lentigines. However, it is possible that the BRAF mutations could develop as a later event in the pathogenesis of nevi (115).
Lentigo Simplex. The lentigo simplex, described in (p. 865), the earlier section on Lentigo Simplex and Related Lesions (93). Histologically, these are small (usually <2 mm), and characterized by an increased number of nevoid melanocytes, present in contiguity with one another near the tips and sides of elongated rete ridges. This pattern, characteristic of lentigines, is therefore described as “lentiginous melanocytic proliferation.” The lack of nests at the histologic level distinguishes the lentigo from a nevus, by definition. However, transitional forms between a simple lentigo and a lentiginous junctional nevus (a lentigo with a few nests) are commonly observed, and the two histologic “entities” are indistinguishable clinically, giving rise to the term “nevoid lentigo” (116) or “jentigo” (96). We prefer the term “lentiginous junctional nevus” for these very common lesions (80) (Fig. 28-5).
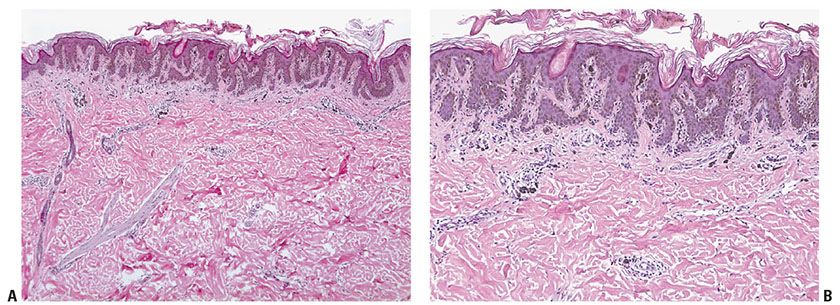
Figure 28-5 A: Lentiginous junctional nevus. Most examples are less than 4 mm, usually about 2 to 3 mm in diameter. A dermal component, if present in a lentiginous nevus, lies in the center of the lesion and the epidermal component extends beyond its “shoulder.” B: In the epidermis, single cells and nests of nevus are arranged near the dermal–epidermal junction near the tips and sides of elongated rete ridges (a “lentiginous” pattern). There is minimal or no atypia. There is no “continuous” proliferation of melanocytes in the suprapapillary regions of the epidermis between the rete ridges. These architectural features are repeated but exaggerated in dysplastic nevi, which, in addition, exhibit mild-to-moderate random cytologic atypia, and more conspicuous stromal reactions.
Junctional Nevus. In a junctional nevus, nevus cells may lie in well-circumscribed nests either entirely within the lower epidermis or bulging downward into the dermis but still in contact with the epidermis, perhaps in the process of “dropping off” to form a compound nevus. The nevus cells in these nests generally have a regular, rounded to cuboidal appearance, although they are occasionally spindle-shaped. In addition, varying numbers of diffusely arranged single nevus cells are seen in the lowermost epidermis, especially in the basal cell layer. In many lesions, single cells are about as common as nests, recapitulating the histology of a simple lentigo. Such lesions in our practice are termed “lentiginous junctional nevi” (Fig. 28-5). Varying amounts of melanin granules are seen in the nevus cells. Some of the nevus cells, on staining with silver, show dendritic processes containing melanin granules, making them indistinguishable from melanocytes, but in general the degree of dendritic differentiation is markedly reduced compared to melanocytes. The nested and single melanocytes are arranged mainly at the tips and sides of rete ridges; “continuous” proliferation of single cells between the rete, confluence of nests, or pagetoid extension of cells into the suprabasal epidermal layers, may be architectural indicators of dysplasia or evolving in situ melanoma.
Although nevus cells only occasionally penetrate into the upper layers of the epidermis (“pagetoid scatter”), aggregates of melanin granules may be seen in the stratum corneum in deeply pigmented junctional nevi. Often, the rete ridges are elongated as in lentigo simplex (p. 868) and single cells as well as nests of nevus cells are seen at the bases of the rete ridges. Not infrequently, as in lentigo simplex, the upper dermis contains an infiltrate of melanophages and mononuclear cells. These lesions that combine features of lentigo simplex and junctional nevus are exceedingly common, and as indicated above, may be termed “lentiginous junctional nevi”. Lesions with these features but larger than 5 mm clinically or 4 to 5 mm in a histologic section often prove to have cytologic atypia and to be dysplastic nevi (p. 896).
In children, some junctional nevi may show considerable cellularity with some degree of cellular enlargement, pleomorphism, and pagetoid cells above the basal layer. They may also often show fine dusty melanin particles and a dense inflammatory infiltrate (117). Some of these lesions may represent Spitz nevi (p. 878) or dysplastic nevi (p. 897). Others may correlate with a tendency for ordinary junctional nevus cells to be enlarged, or epithelioid, in younger individuals. The small size of the lesion, the sharp lateral demarcation, the lack of severe or uniform atypia and of mitoses, and the fact that in children melanomas are very rare, are features that help in the distinction from melanoma. However, if the criteria mentioned above are present, the diagnosis of melanoma should be considered, even in a child.
Compound Nevus. Clinically, a compound nevus is a pigmented papule (Fig. 28-6A) or a plaque. In most nondysplastic compound nevi, there is no adjacent macular component. Histologically, a compound nevus possesses features of both a junctional and an intradermal nevus. Nevus cell nests are present in the epidermis, as well as appearing to “drop off” from the epidermis into the superficial dermis and in many lesions, the reticular dermis (Fig. 28-6B). This time-honored theory of “abtropfung” or dropping off of nevus cells proposed by Unna has been challenged by the finding that junctional nevi are at least as common in adults as in children (118). Nevus cells in the upper, middle, and lower dermis may present characteristic morphologic variations called types A, B, and C, respectively (51,119). Usually, the Type A nevus cells in the upper dermis are round to cuboidal, show abundant cytoplasm containing varying amounts of melanin granules, and tend to form nests. Type A cells with especially abundant cytoplasm, as may occur in children and young adults, may be termed “epithelioid cells” (Fig. 28-6C). Melanophages are occasionally seen in the surrounding stroma. The cells in the mid-dermis usually are type B cells; they are distinctly smaller than the type A cells, display less cytoplasm and less melanin, and generally lie in well-defined aggregates or cords. They may to some extent resemble lymphoid cells (Fig. 28-6D). Type C nevus cells in the lower dermis tend to resemble fibroblasts or Schwann cells, because they are usually elongated and possess a spindle-shaped nucleus. They often lie in strands and only rarely contain melanin (Fig. 28-6E). Occasionally, they form aggregates that resemble Meissner corpuscles. Occasional nevi show abnormal stratification within the deeper dermis of the otherwise benign type A nevus cells, resulting in the designation of “inverted type A nevus.”
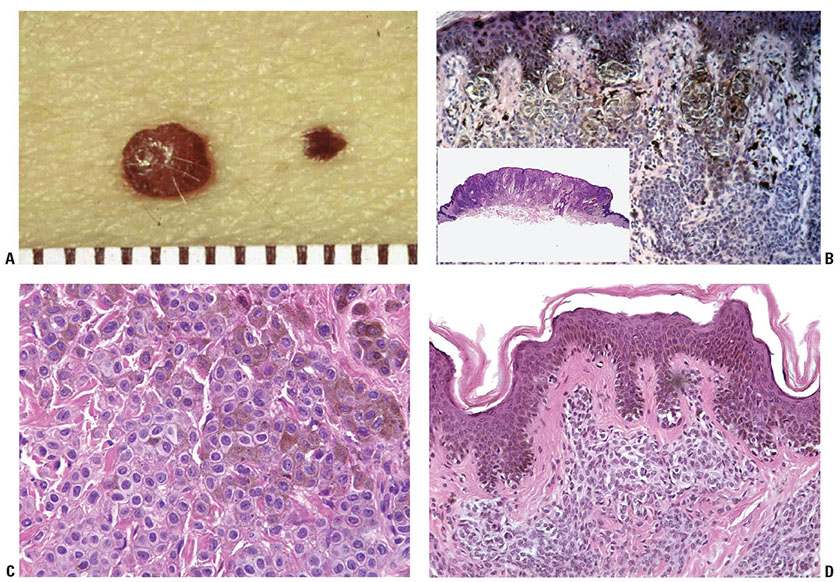
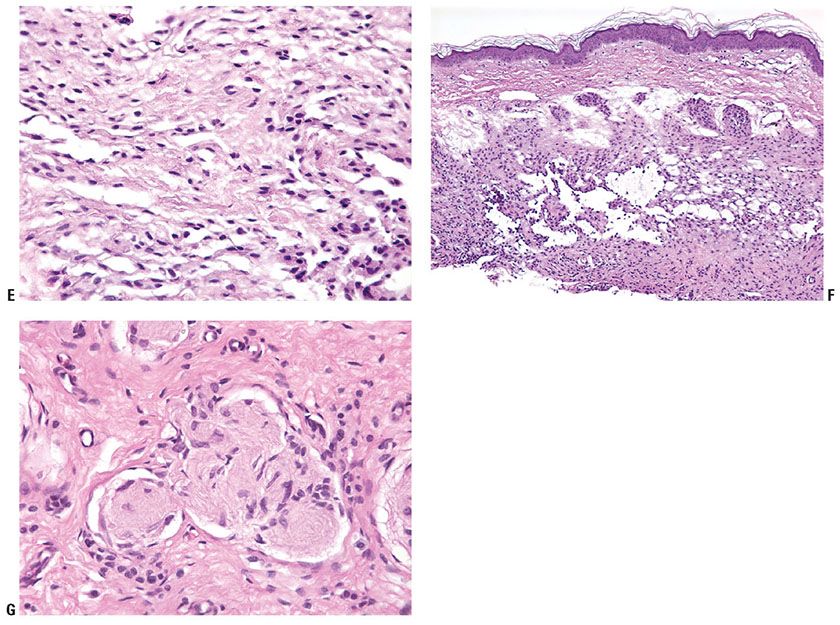
Figure 28-6 A: Compound nevi. Each lesion is a true papule without any adjacent macular component. The lesion on the right is a pigmented compound nevus. The lesion on the left has little pigment, and clinically is a predominantly dermal nevus. B: Histologically, the lesion is a true papule without an adjacent junctional component (inset). Nests of nevus cells are present at the dermal–epidermal junction. It is believed that these nests become separated from the epidermis, to lie in the dermis, piling upon one another in an “accretive” pattern of growth, and resulting in gradual elevation of the epidermis above its original position to form the papule. Pigment is present mostly in junctional and superficial dermal nevus cells. C: Dermal nevus with type A nevus cells. The “type A” cells have visible cytoplasm that is in contact with that of neighboring nevus cells. The nuclei are small, without atypia or prominent nucleoli. There are no mitoses. D: Lentiginous compound nevus. In this, as in many nevi, nests are admixed with single cells in the junctional component, a lentiginous pattern. The cells in the dermis are small lymphocyte-like “Type B” cells. E: Type C dermal nevus cells at the base of a nevus. The cells at the base of a nevus tend to be spindled in shape and tend to have collagen between the individual cells. If they extend into the reticular dermis, they tend to “disperse” as individual cells among the superficial collagen fibers, a pattern that is also characteristic of Spitz nevi. F: Dermal nevus with “pseudo-lymphatic spaces.” The type B nevus cells at the top of the lesion have small nuclei and scant cytoplasm, reminiscent of lymphocytes. The spaces are a common artifact in dermal or compound nevi. They may simulate lymphatic invasion of a melanoma, but are completely benign. The cells at the base of the lesion are predominantly type C cells. G: Neurotized dermal nevus cells at the base of a dermal nevus. The structures at the base of a “neurotized” dermal nevus may be reminiscent of nerve fibers or neural organs such as Wagner–Meissner corpuscles.
The decrease in cell size, melanization, and progression from nests to cords to more neuroid spindle cells with dermal descent seen in nevi is often referred to as maturation and is regarded as evidence of benignity, because the size of the cells in a melanoma usually does not decrease with depth. The process of nevus cell maturation has alternatively been regarded as one of senescence or atrophy (120), perhaps driven by the tumor suppressor p16, which is activated in response to the effects of an activated oncogene such as BRAF or NRAS in a phenomenon termed “oncogene-induced senescence”(121). If dermal nevus cells are confined to the papillary dermis, they often retain a discrete or “pushing” border with the stroma. However, nevus cells that enter the reticular dermis tend to disperse among collagen fiber bundles as single cells or attenuated single files of cells. This pattern of infiltration of the dermis differs from that in most melanomas, where groups of cells tend to dissect and displace the collagen bundles in a more “expansive” pattern (122). Lesions where nevus cells extend into the lower reticular dermis and the subcutaneous fat or are located within nerves, hair follicles, sweat ducts, and sebaceous glands, may be termed “congenital pattern nevi” because they share morphologic features with congenital nevi but are not necessarily present at birth (123) (p. 888).
Intradermal Nevus. Intradermal nevi show essentially no junctional activity. The upper dermis contains nests and cords of nevus cells. Multinucleated nevus cells may be seen, in which small nuclei lie either in a rosette-like arrangement or close together in the center of the cell. These nevus giant cells differ significantly in appearance from the irregularly and even bizarrely shaped giant cells that are seen frequently in Spitz nevus and occasionally also in melanoma. As a result of shrinkage during tissue processing, clefts may form between some nests of nevus cells and the surrounding epidermis as well as stroma, the latter leaving a defect that simulates a lymphatic space and thus mimics lymphatic invasion (Fig. 28-6F) (124).
Whereas the nevus cell nests located in the upper dermis often contain a moderate amount of melanin (particularly type A cells), the type B and C nevus cells in the mid-portion and the lower dermis rarely contain melanin. Type C cells appear spindle-shaped, are arranged in bundles, and are embedded in collagenous fibers having a loose, pale, wavy appearance similar to that of the fibers in a neurofibroma, resulting in a “neurotized nevus.” Such formations have been referred to as neuroid tubes. In other areas, the nevus cells lie within concentrically arranged, loosely layered filamentous tissue, forming so-called nevic corpuscles that resemble Meissner tactile bodies (Fig. 28-6G). Neurotized nevus cells express the marker S100A6 protein, a form of S100 found in Schwann cells, supporting the hypothesis that maturation in these lesions recapitulates some features of Schwann cell differentiation (125).
Occasional intradermal nevi are devoid of nevus cell nests in the upper dermis and contain only spindle-shaped nevus cells embedded in abundant, loosely arranged collagenous tissue. These nevi may be referred to as neural nevi. The differentiation from a solitary neurofibroma may be difficult in routinely stained sections, but distinction might be possible with an immunohistochemical technique employing myelin basic protein, which is positive only in neurofibroma (126) (see Histogenesis). Neurofibromas also tend to contain small nerve twigs and axons that can be highlighted with glial fibrillary acidic protein (GFAP) staining (127), a feature not typical of neurotized nevi.
Some intradermal and, less commonly, compound nevi show hyperkeratosis and papillomatosis, which may be associated with a lace-like, downward growth of epidermal strands and with horn cysts. Such nevi resemble seborrheic keratoses in their epidermal architecture. In other instances, large hair follicles are observed. Rupture of a large hair follicle may manifest itself clinically in an increase in the size of the nevus associated with an inflammatory reaction, leading to clinical suspicion of a melanoma. Histologic examination in such instances shows a partially destroyed epidermal follicular lining with a pronounced inflammatory infiltrate containing foreign body giant cells as a reaction to the presence of keratin in the dermis. Occasionally, intradermal nevi contain scattered large fat cells within the aggregates of nevus cells. This could represent a regressive phenomenon in which fat cells replace involuting nevus cells, or alternatively adipocyte metaplasia within the stroma of the nevus.
Some intradermal nevi, including variants of spindle and epithelioid cell nevi, induce marked deposition of coarse, sometimes hyalinized collagen. Such nevi are described as sclerosing variants. This change occasionally may confound diagnosis, but is not of biological significance.
In occasional otherwise typical dermal nevi (or in the dermal component of compound nevi), rare mitoses are found in the dermis. This phenomenon has been described in children (117), and in association with pregnancy (128). In a recent comprehensive study, of 1,041 benign nevi, 82 (7.9%) contained one or more mitoses, most of which were in the papillary dermis, in compound nevi. Only three cases contained three mitoses (129). If there are no other indicators of malignancy, we report these cases as “nevi with mitoses,” and generally recommend complete excision. The possibility of nevoid melanoma (see section “Nevoid Melanoma, p. 927”) should be seriously considered in such lesions (130).
Histogenesis of Acquired Melanocytic Nevi. For many years, Masson’s theory of the dual origin of nevus cells was widely accepted (51). He believed that the nevus cells in the upper dermis developed from epidermal melanocytes, while the nevus cells in the lower dermis developed from Schwann cells, as suggested by the frequent presence of nerve-like structures in the latter. The fact that both melanocytes and Schwann cells are derived from the neural crest seemed to support Masson’s view, as did the presence of a nonspecific cholinesterase reaction in both deep nevus cells and Schwann cells, and the absence of melanin in the deep nevus cells (131). However, in favor of a melanocytic origin of these deep dermal nevus cells was the presence of melanosomes with dopa-oxidase activity even in deeply situated nevus cells that had a neuroid appearance on light microscopy (132). An electron microscopic examination of neuroid structures in nevi revealed that these “nevic corpuscles” contained no Schwann cells or axons, but were instead composed exclusively of cells that contain premelanosome-like dense bodies in their perikaryon (133). Furthermore, myelin basic protein has been found to be regularly present by immunoperoxidase in Schwann cells and absent in all types of melanocytic nevi (126). In addition, “melanocyte-specific markers” like Melan-A/MART-1, HMB-45, tyrosinase, and others are often positive in nevi but typically absent in neural cells and neurofibromas (134).
Another point in favor of a single origin of the nevus cell is to be found in the life cycle of nevi. Although there are exceptions, most nevi appear in childhood, adolescence, and early adulthood, and, with advancing age, there is a progressive decrease in the number of nevi (135). The evolution and involution of nevi correlate with their histologic appearance. Junctional proliferation of nevus cells is present in almost every nevus in children, but decreases with age. Intradermal nevi, by contrast, are most unusual in the first decade of life, and their proportion increases progressively with age. The incidence of fibrosis, fatty infiltration/metaplasia, and neuroid changes increases with age. Thus, the formation of cylindrical neuroid structures represents the end stage of differentiation and not a source of origin of intradermal nevi (136).
Concerning the relationship between epidermal melanocytes and nevus cells, some authors believe that these two types of cells have a different embryologic genesis, with the nevus cell originating from a neural crest precursor cell referred to as a nevoblast (137). Most authors regard the two cell types as identical, however (138). It would seem that the morphologic features by which nevus cells differ from melanocytes, such as the absence of dendrites as seen by light microscopy, their arrangement in cell nests, their larger size, and their tendency to retain pigment, are secondary adjustments of the cells. Electron microscopy has shown that the fine structure of nevus cells is comparable to that of epidermal melanocytes (139) (EM 46, 47). Cultured nevus cells, whether derived from congenital or acquired lesions, have been found to be highly dendritic, as are epidermal melanocytes (138). Immunohistochemical markers such as MITF are also similarly expressed between the two cell types (140). In conclusion, it seems established that nevus cells differ from Schwann cells and are benign neoplastic variants of melanocytes.
The molecular pathology of nevi is beginning to be understood. In a recent study of the BRAF oncogene, which had previously been found to be mutated in a high percentage of metastatic melanomas (141), mutations resulting in the V600E amino acid substitution were found in 68% of melanoma metastases, 80% of primary melanomas, and unexpectedly, in 82% of nevi (142). Activating mutations of the oncogene NRAS (mutually exclusive with BRAF mutations) have also been described in melanoma-associated nevi and in congenital nevi (143–146). The BRAF mutations have been found to be clonal by sensitive genetic methods and by also using an antibody specific for the mutated gene, suggesting that these mutations are likely to be early events in melanocytic neoplasia (147). These data suggest that mutational activation of the RAS/RAF/MAPK mitogenic pathway in nevi is a critical step in the initiation of melanocytic neoplasia but alone is insufficient for melanoma tumorigenesis (142). High levels of the tumor suppressor gene product p16INK4 in benign nevi may represent the mechanism whereby the cell cycle remains regulated in nevi, even in the presence of activating oncogene mutations (121,148–150).
Principles of Management of Nevi. Common nevi and related lesions generally do not require active therapy. Lesions are often excised for cosmetic reasons, and clinically atypical lesions are excised to rule out melanoma. An increased number of nevi, especially large nevi, is also a risk factor for future development of melanoma, and may prompt consideration of surveillance.
Balloon Cell Nevus
Balloon cell nevi are histologic curiosities that possess no clinical features by which they can be differentiated from other nevi. They are quite rare.
Histopathology. Balloon cells may be seen within the epidermis singly or in groups, or may be absent from the epidermis. In the dermis, they lie arranged in lobules of varying size often with an admixture of ordinary nevus cells, and often with transitional forms between the ordinary and ballooned nevus cells (Fig. 28-7). The balloon cells may be multinucleated, and are considerably larger than ordinary nevus cells. Their nuclei are small, round, and usually centrally placed. Their cytoplasm appears empty, finely granular, or vacuolated, often with a few small melanin granules. There may be melanophages that are solidly packed with pigment. Stains for lipids, glycogen, and acid or neutral mucopolysaccharides are negative in the balloon cells. Electron microscopic examination reveals in balloon cells numerous large vacuoles formed by enlargement and coalescence of degenerating melanosomes (151). Balloon cell nevus is differentiated from balloon cell melanoma by the usual criteria (p. 924). The large adipocytes present in some intradermal nevi as a result of fatty infiltration or stromal metaplasia (p. 872) differ from balloon cells by routine histology by having a flattened nucleus located at the periphery of the cell. In the differentiation from clear cell hidradenoma and other clear cell tumors, the absence of PAS-positive glycogen and keratin in balloon cell nevus might be helpful; balloon nevus cells also stain for S100 protein, and although eccrine neoplasms (and adipocytes) may also express this marker, they will also usually be positive for Melan-A/MART-1, and negative for keratin markers.
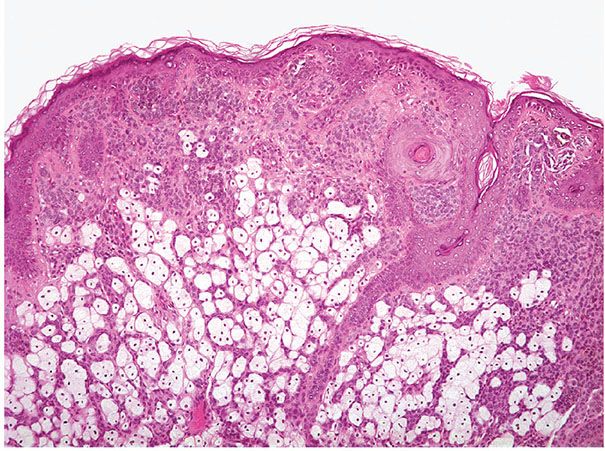
Figure 28-7 Balloon cell nevus. Large cells with pale cytoplasm are admixed with mature nevus cells. There is no high-grade atypia or mitotic activity.
Nevi of Special Sites
There is apparent variation in the morphology of common nevi by site. In a study of Australian schoolchildren, gender differences in nevus density on the back and lower limbs were similar to gender differences for melanoma, with back lesions favoring males, and lower extremity lesions favoring females. Small nevi (2–4 mm) were most dense on the arms, whereas large nevi (≥5 mm) were most dense on the posterior trunk where they were related to age, male sex, and freckling. The findings were considered to support the hypothesis of site-specific differences in nevus proliferative potential (152). Nevi in certain locations may exhibit features that are unusual in the vast majority of common nevi on the trunk, limbs, or face and scalp.
Clinical Summary. These “special site nevi” have been best defined in acral locations (palmar and plantar, and subungual nevi), and in genital skin. Nevi in skin flexural areas (ear lobe, axilla, umbilicus, inguinal creases, pubis, scrotum, and perianal area) may also show unusual features, similar to those to be described below for acral nevi (153). Recently, the skin of the ears, the skin of the breast, and the skin of the scalp in adolescents have been added to this seemingly ever-expanding list of “special” sites (154–157). In general, the “special” features of the nevi are seen in only a minority of the nevi from these sites; the other nevi are quite unremarkable. Other names for nevi of special sites include: atypical genital nevi (AGN), atypical melanocytic nevus of the genital type (AMNGT), melanocytic acral nevus with intraepithelial ascent of cells (MANIACs), acral-lentiginous nevus of plantar skin, atypical nevi of the scalp, and special site nevi (158). In a study of nevi with mitoses, the highest incidence of mitoses among body site was observed in special sites (10.9%), which included genitals, perineum, groin, and acral regions, perhaps supporting the notion that these nevi are more “active” than others (129).
Nevi of Acral Skin
Clinical Summary. Melanocytic nevi are present on the skin of the palms or soles in as many as 4% to 9% of the population (159). Clinically, they are usually small, symmetrical, well-circumscribed brown macules, with a tendency to dark ridging along dermoglyphics that can be clinically striking and may impart a seemingly irregular border. They are usually stable, and are more often junctional than are nevi of the trunk.
Histopathology. Acral nevi tend to be more cellular than most common nevi, and the nevus cells may be arranged in predominantly lentiginous rather than nested patterns in the epidermis. Pagetoid proliferation (“pagetoid melanocytosis”) of lesional nevus cells in the epidermis above the basal layer is relatively common in benign acral nevi (160–163). These features may perhaps account for recommendations in the older literature to remove acral nevi because of suspicion of melanoma. However, there is no evidence that these lesions, when devoid of true dysplasia, are common precursors or risk markers for acral melanomas.
Acral nevi may simulate and must be differentiated from melanoma, and this may be difficult especially in small biopsies. Clemente and colleagues studied a series of acral nevi and identified a subset that they termed acral-lentiginous nevi (161). The distinctive features of these nevi compared to other acral non-lentiginous nevi included several features also seen in acral-lentiginous melanomas: poor lateral circumscription, elongation of rete ridges, continuous junctional proliferation of melanocytes, the presence of scattered melanocytes within the upper epidermis, and the presence of junctional melanocytes with abundant pale cytoplasm and round to oval, sometimes hyperchromatic, nuclei with prominent nucleoli. Cytologic atypia is not marked in most acral nevi of the palms and soles. Compared to acral melanomas, the lesional cells in acral nevi preferentially proliferate in the crista profunda limitans, which is an epidermal rete ridge underlying a surface furrow. As a result, regular melanin columns are produced beneath these surface furrows (164).
In compound acral nevi, the nevus cells in the dermis, unlike melanoma cells, mature to the lesional base. There is no high-grade uniform cytologic atypia, no extensive and high-level pagetoid melanocytosis in the epidermis, and there are no mitoses (Fig. 28-8). There may be patchy lymphocytes and occasional melanophages in the dermis.
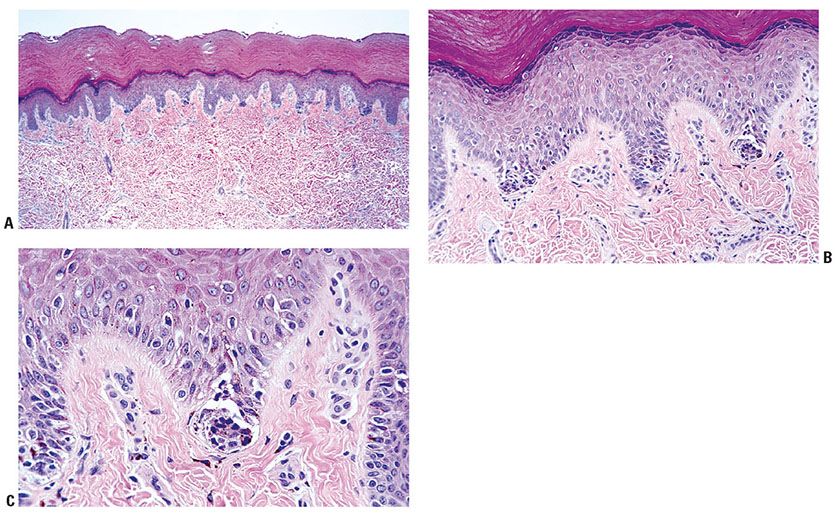
Figure 28-8 Acral-lentiginous nevus. A: The lesion is small, quite well-circumscribed, and entirely contained within the biopsy specimen. B: Nests of nevoid to small epithelioid melanocytes predominate near the dermal epidermal junction. They tend to be small, evenly spaced, and discrete. C: Although a few lesional cells may be present above the junction in a “pagetoid” pattern, there is no severe uniform atypia or mitotic activity, and there is no continuous proliferation of single cells between the nests.
Some of the features of acral nevi may suggest dysplastic nevus. However, the rete ridge pattern of keratinocytes in most acral nevi does not show the uniform elongation with occasional accentuation of anastomosing rete that characterizes dysplastic nevi, the complete array of stromal changes of dysplastic nevi are not observed in most acral nevi, and atypia of melanocytes, as a rule, is minimal or absent.
In case of doubt, it may be appropriate to evaluate the patient’s other nevi, especially if there is a family or personal history of melanoma. An acral-lentiginous lesion that extends to specimen borders should be evaluated carefully, and clinicopathologic correlation should be obtained to ensure that the specimen does not represent the periphery of a larger lesion. In such cases it may be judicious to recommend complete excision, to rule out additional pathology, and to preclude persistence or recurrence of the lesion. This is also recommended for any acral-type nevus, which in addition to exhibiting site-related architectural features, also shows significant cytologic atypia. Finally, because a nevus is acral does not preclude the unusual possibility that it is also dysplastic, but this diagnosis must rely on the presence of cytologic and stromal criteria for dysplastic nevus in addition to those commonly encountered in nevi of acral sites.
Subungual Nevi and Melanonychia Striata (Longitudinal Melanonychia)
Clinical Summary. Melanonychia striata (see also Chapter 19) refers to a pigmented band extending in the long axis of the nail. Such bands are common in Blacks and Asians and therefore are regarded as normal (165). However, the sudden appearance of melanonychia striata is cause for concern, particularly in Whites, often requiring a punch biopsy of the nail matrix (166). An exception is the melanonychia striata seen in the Laugier–Hunziker syndrome, which may affect one, several, or all fingernails in association with pigmented macules of the lips or the buccal mucosa. This type of melanonychia is always benign (165,167).
For carrying out the biopsy, longitudinal bilateral releasing incisions are made along the medial and lateral sides of the posterior nail fold. Next, the entire posterior nail fold is reflected proximally to expose and make visible the very end of the pigmented streak, which is usually within the matrix of the nail. Then the biopsy specimen is taken with a 3- or 4-mm punch through the nail plate and matrix down to the phalangeal bone (168).
Histopathology. Histologic examination in most instances shows merely basal cell layer hyperpigmentation without an obvious increase in the number of melanocytes. Such lesions have been termed “melanotic macules” (169) or “nail matrix activation,” referring to an increased production of melanin with a normal number of melanocytes (170). In a study of 18 cases, 10 were melanotic macules, 1 case was melanoma in situ, another showed keratinocytic atypia, and 3 were subungual hemorrhages (171). In other cases, a junctional nevus or a compound nevus of acral type as described above, or an acral-lentiginous melanoma, either in situ or invasive, may be found. The junctional or compound nevi tend to be of the lentiginous acral nevus pattern described above. Longitudinal melanonychia may also be associated with pigmented Bowen disease (166). Exclusion of a biologically significant lesion should involve adequate sampling of the nail matrix, and when there is doubt as to whether a proximal site of origin for the clinical lesion is included, additional sampling should be considered. Longitudinal melanonychia in children is almost always benign: in a study of 40 cases in children under the age of 16, the histologic diagnosis was nevus in 19 cases (junctional in 17 cases and compound in 2), lentigo in 12 cases, and simple hyperpigmentation (“functional melanonychia) in 9. None of the patients in this series had melanoma (172). Subungual melanomas, though very rare, do however occasionally occur in children; for example, the diagnosis in a 13-year-old boy was supported by comparative genomic hybridization findings (173).
Nevi of Ear Skin
Sometimes considered to be an acral site, the ear differs in being heavily exposed to solar radiation in men, but not usually in women, and is the single most common site for melanoma in men after correction for unit area. Nevi of the ear may show somewhat similar features to acral nevi, though often with greater atypia (158). In one study, pagetoid spread, moderate-to-severe cytologic atypia, and prominent nucleoli were prominent in 50% to 60% of 21 auricular nevi. None of these cases showed mitoses or apoptotic melanocytes (156). In another study of 101 lesions, nests varied in size and shape and were located between rete ridges in most cases, while in about 40% there was poor circumscription, lateral extension of the junctional component beyond the dermal component, and elongation of rete ridges with bridging between them. About 25% had uniformly large melanocytes with large vesicular nuclei without prominent nucleoli, and abundant pale, finely granular cytoplasm (155). These are features that could overlap considerably with melanoma. Features that tended to diminish concern for melanoma in these lesions included symmetry, lack of nuclear pleomorphism, the absence of pagetoid scatter of melanocytes, and maturation of the dermal component in compound lesions. The lesions in this study did not exhibit a tendency to recur. Nevertheless, we would recommend complete excision for any lesion that exhibits these features to any significant degree, especially if they are present in sun-damaged skin of an older subject.
Nevi of Genital Skin
Clinical Summary. Clinically unremarkable nevi located on or near genital skin may present with histologic features that simulate some aspects of melanomas. The lesions have no clinical significance except the possibility of diagnostic error. They have been termed “AMNGT,” and are most often seen on the vulva of young (premenopausal) women, but also may be seen on perineal skin (174–176). Similar lesions also occur uncommonly on the male genitalia (177). The lesions are often removed incidentally. They are typically symmetrical papular lesions, usually less than 1 cm in diameter, and uniformly pigmented with discrete well-circumscribed borders. The atypical features appear to represent a histologic curiosity seen in a minority of vulvar nevi. In a comparative histologic study of vulvar and common nevi, most of the vulvar lesions were unremarkable (178). Vulvar nevi themselves are quite uncommon; in patients in a gynecology practice, the prevalence was only 2.3% (179). An interesting variant pattern of nevi has been described in association with lichen sclerosus et atrophicus, most commonly on skin of the vulva, perineum, or rarely elsewhere. These nevi had features in common with persistent (“recurrent”) melanocytic nevi and can mimic malignant melanoma. This “activated” melanocytic phenotype seen in lichen sclerosus–associated melanocytic nevi suggests a stromal-induced change (175). The clinical differential diagnosis for atypical genital nevi also includes early “genital lentigines,” as described above.
Histopathology. The scanning magnification impression is typically that of a small, well-circumscribed papular lesion composed of nevus cells arranged in clusters in the papillary dermis, and arranged mainly in nests in the epidermis where the cells usually do not extend beyond the shoulder of the dermal component, as typically occurs in compound dysplastic nevi (Fig. 28-9). The epidermis occasionally is irregularly thickened, resulting in an asymmetrical silhouette. The nevus cells may be large, with prominent nucleoli, and abundant cytoplasm containing finely divided (“dusty”) melanin pigment. The nests tend to be variable in size, shape, and position, originating from the sides as well as the tips of rete, and often oriented parallel to the surface and sometimes confluent. In a study of 56 lesions in 55 female patients with a median age of 26 years, the dominant histologic feature was a lentiginous and nested junctional component composed of prominent round or fusiform nests, often showing retraction artifact and/or cellular dyscohesion (180). Cytologic atypia was mild in 11 cases (20%), moderate in 34 (60%), and severe in 11 (20%). Ten cases (18%) had focal pagetoid spread, generally to a low level of the epidermis. The atypical junctional melanocytic proliferation was associated with a large common dermal nevus component that was dominant in 26 cases (46%). Adnexal spread (46%) and nuclear atypia of melanocytes in the superficial dermis (39%) were relatively common, while dermal mitoses (7%) were uncommon and maturation was present in all cases. A broad zone of dense eosinophilic fibrosis within the superficial dermis was a frequent finding (41%). Only one lesion recurred after the initial excision, and was re-excised with no further eventuality.
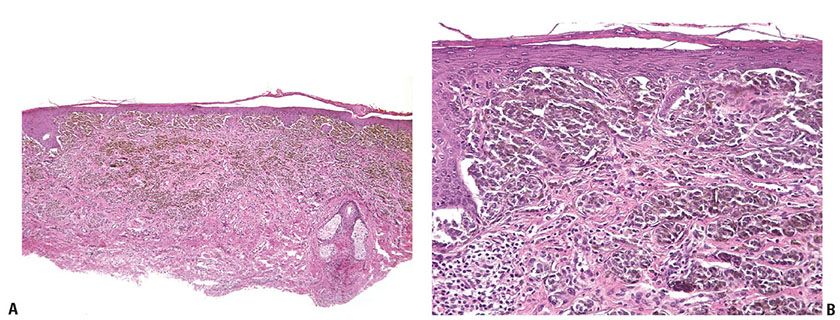
Figure 28-9 A: Nevus of genital skin. Nevi on genital skin may exhibit atypical features at high power, but are usually relatively small and symmetrical and thus benign in their appearance at scanning magnification, and also clinically. B: Atypical features in genital nevi may include nests that tend to be large and confluent, and vary a good deal in size and shape, and include cells that tend to be large, with macronucleoli, and dyshesive from one another. Mitoses are rare or absent, and there is no adjacent in situ or microinvasive radial growth phase component.
Some of the features seen in atypical vulvar nevi may arouse a suspicion of melanoma. However, the lesions are comparatively small and well-circumscribed, without significant junctional proliferation of atypical melanocytes beyond the major dermal component, as is seen in most mucosal melanomas. Moreover, there is little or no pagetoid spread of single or nested melanocytes into the epidermis, there is no necrosis, and usually no ulcer, and importantly there is little or usually no mitotic activity in the dermis (178). Stromal patterns more characteristic of melanoma (diffuse fibroplasia) or dysplasia (concentric fibroplasia) are generally lacking in these lesions (176). The diagnosis of melanoma in vulvar skin should be made with caution (but is sometimes unavoidable) in a premenopausal woman. Occasional vulvar nevi do exhibit high-grade cytologic atypia; however, and although the biological behavior of such lesions in young to middle-aged women has not been fully studied, it is prudent to modestly excise such lesions to prevent local persistence or recurrence in scar tissue.
In an apparently unrelated phenomenon, atypical nevi have been described in association with lichen sclerosus et atrophicus. These lesions resemble persistent or recurrent melanocytic nevi (so-called “pseudomelanoma”; see section “Recurrent Nevus (Pseudomelanoma)”, p. 894 (175).
Nevi of Flexural Sites and of Breast Skin
On the basis of histologic findings, it has been suggested that nevi in flexural sites may differ histologically from those at other sites, in general resembling the appearances of nevi of genital skin, and potentially simulating melanoma. In a study of 40 melanocytic nevi of flexural sites (axilla, umbilicus, inguinal creases, pubis, scrotum, and perianal area), 22 of them had “a nested and dyshesive pattern” similar to the genital pattern nevi. This pattern was characterized by the confluence of enlarged nests with variation in size, shape, and position at the dermo-epidermal junction and by the diminished cohesion of melanocytes (153).
Nevi of breast skin may show similar features, including intraepidermal melanocytes above the basal layer, melanocytic atypia, and dermal fibroplasia (157). Cytologic atypia is said to be somewhat greater than in other special site nevi (158), and thus lesions may resemble those typical of genital skin in young women (see above).
These changes in flexural and breast nevi, like those in other “special sites” should not be over-interpreted as indicative of melanoma; by the same token, authentic dysplastic nevi and melanomas may occur in these sites and should not be underdiagnosed (181).
Nevi of Scalp Skin
In a study of 229 nevi of the scalp from all ages, about 10% of the nevi from adolescents had atypical cytologic and architectural aspects that included the presence of large bizarrely shaped nests scattered disorderly along the junction with follicular involvement, pagetoid spread of cells above the junction, and a discohesive pattern of the melanocytes in the nests. Mild cytologic atypia was present but less significant. These atypical features were not found in scalp nevi from adults or younger children. Although these lesions were considered to be benign, complete excision with conservative margins was recommended (154). In another study of 59 lesions, the prototypical special site scalp nevus was considered to be one that contains poorly cohesive melanocytes arranged in large nests positioned both along the sides of rete and between rete ridges, housing random melanocytes with large nuclei and abundant pale cytoplasm, and exhibiting adnexocentricity and splaying of melanocytes through the reticular dermis. Overlap features with Clark nevi including nests that bridge rete, lateral extension past the dermal component, and papillary dermal fibroplasia were also common to these lesions (182).
Special Site Nevi with Complicating Features
The above-mentioned features of nevi from special sites may be additionally problematic when combined with age-related benign nevus cell enlargement (so-called epithelioid cell change that often occurs in the superficial component of nevi from children), inflammation (as in halo-type regression), and trauma. Thus, just as it is important not to overlook true dysplasia in nevi, it is also imperative to consider components of atypia that may contribute to potential overdiagnosis of dysplasia or melanoma. Careful correlation of histologic changes with clinical parameters (age, halo-like changes, previous trauma, or biopsy) is of assistance in this regard.
Principles of Management of Special Site Nevi
Consideration of complete surgical excision is appropriate for all nevi of special sites, to allow for complete pathologic examination, and minimize any chance of persistence, recurrence, or progression of the lesion (158). In selected cases, follow-up may be appropriate.
In most, if not all, of the studies of special site nevi, correlations with other clinicopathologic variables including family history and the nature and characteristics of other melanocytic lesions present in these individuals were not available. Melanoma and authentic dysplastic nevi both may occur in all or most of these sites, most often in adults and rarely in older children and adolescents. The descriptions of special site nevi in these various sites are helpful and should lead to a cautious approach to diagnosis of these lesions. In some cases, particularly in the distinction between a special site nevus and a dysplastic nevus or a melanoma, it is appropriate to express uncertainty. If there is uncertainty between a nevus of special sites and a dysplastic nevus, but no concern for melanoma, a term such as “compound or junctional nevus with atypical features” might be used. In a note, the differential diagnosis can be expressed.
An individual in whom such a lesion is diagnosed should likely be offered a complete skin exam, and a detailed family history should be taken. Especially if there are other clinically atypical nevi and/or a family or personal history of melanoma, periodic skin surveillance may be an appropriate consideration, whereas if the atypical lesion is an isolated phenomenon, it likely has no clinical significance except as a potential simulant of melanoma. In a case where the differential diagnosis includes melanoma, a term such as “superficial atypical melanocytic proliferation of uncertain significance (SAMPUS)” can be used if there are atypical cells in the dermis, or “intraepidermal atypical melanocytic proliferation of uncertain significance” (“IAMPUS”)” if the atypical proliferation is entirely confined to the epidermis (p. 916). Clinicians can then assess the level of uncertainty in determining their management recommendations.
Spitz Nevus/Tumor
The Spitz nevus, named after Sophie Spitz, who first described it in 1948 (183), has been known also as benign juvenile melanoma (a term that is archaic and no longer used) and as spindle and (/or) epithelioid cell nevus.
Clinical Summary. The lesion was originally thought to occur largely in children, but it is now well-recognized in young-to-early middle-aged or even older adults (184,185). Rarely, it is present at birth (186). Because of a degree of unpredictability in the behavior of a subset of cases, particularly when lesions occur in adults, the term “Spitz tumor” has been suggested as preferable to “nevus,” except in the most typical cases, especially those in young children (187,188).
The lesion usually is solitary and is encountered most commonly on the lower extremities and face (189). In one study, Spitz nevi were found to predominate on the thighs in persons younger than 40 years of age, while in contrast, on the trunk melanomas were more frequent in persons 40 years of age or older (190). In most instances, the lesion consists of a dome-shaped, hairless, small pink nodule. Most Spitz nevi are small: in 95% of the patients, the size of the tumor is less than 1 cm, and in 75% it is 6 mm or less (191). The color is usually pink because of a paucity of melanin and in some lesions associated vascularization of the stroma, and it is then often diagnosed clinically as a pyogenic granuloma, an angioma, or a dermal nevus. However, it may be tan and, in some cases, brown or even black. Ulceration is seen only rarely, in our experience most commonly in lesions from young children. After an initial period of growth, most Spitz nevi are stable.
In rare instances, multiple tumors are encountered. These may be either agminated (grouped) in one area (192), or widely disseminated (193) (Fig. 28-10A), and occasionally within a nevus spilus. In a case of agminated Spitz nevi, a mosaic pattern of chromosomal translocation was demonstrated in lesional fibroblasts, suggesting a local event in embryogenesis (192). The histopathologic findings can range from those of a classic Spitz nevus to an atypical Spitzoid neoplasm that may be difficult to distinguish from a Spitzoid melanoma. In a recent series of nine patients, 53% of the excised lesions showed no atypical histopathologic features and none had recurred after a reexcision (194).
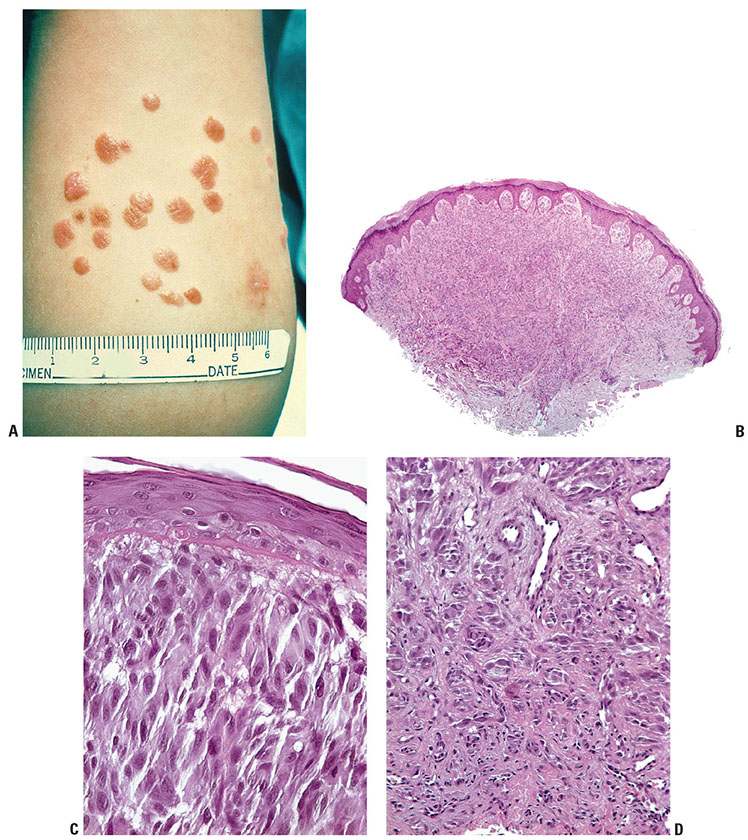
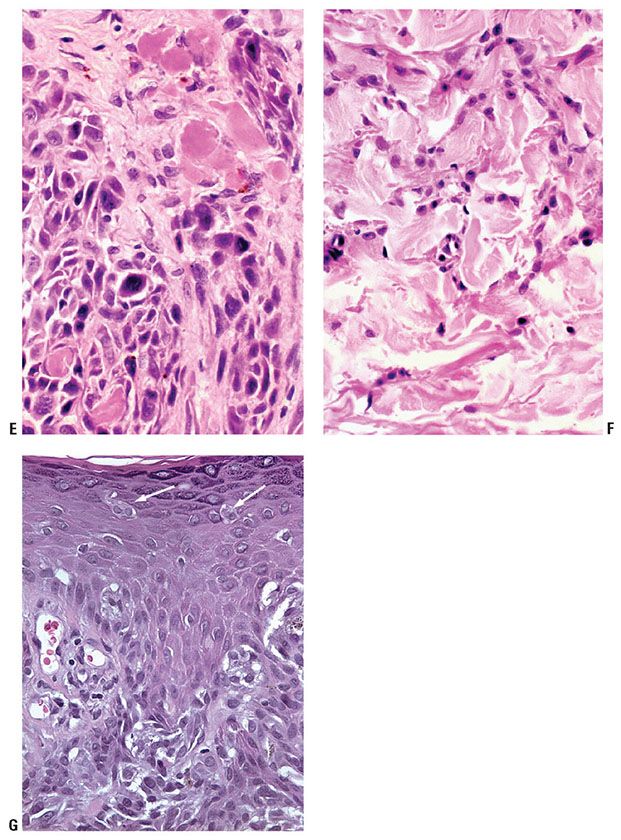
Figure 28-10 A: Agminated Spitz nevi. Most Spitz nevi are single lesions. In this example of agminated, or grouped Spitz nevi, each individual lesion is a characteristic Spitz nevus—relatively small, symmetrical, well-circumscribed tan papules. B: Spitz Nevus. Most Spitz nevi are small, circumscribed, symmetrical papules, at scanning magnification and clinically. This lesion is somewhat larger than most examples. C: Spitz nevi are defined by the presence of large cells, with abundant amphophilic cytoplasm, which may be spindled or polygonal in shape (“large spindle and/or epithelioid melanocytes”). The nuclei in a given Spitz nevus are more homogenous than in most melanomas, usually large but with open chromatin and smooth nuclear membranes, and with prominent nucleoli. D: “Maturation” from larger cells near the surface to smaller cells at the base is an important feature of Spitz nevi. E: Spitz nevus with globoid eosinophilic bodies (“Kamino bodies”). Although not pathognomonic, these structures are highly characteristic of Spitz nevi. F: At the base of a Spitz nevus, the lesional cells become smaller, and become “dispersed” among the reticular dermis collagen fibers. This is the same lesion as D. Note the diminution in size (“maturation”) of the lesional cells at the base. G: Pagetoid melanocytosis. Pagetoid extension of some cells of a Spitz nevus into the epidermis, as seen here (arrows), is not diagnostic of a melanoma if other attributes of melanoma are not observed. This pagetoid melanocytosis is not uncommon at least focally in lesions of young children.
Histopathology. Ackerman’s term “nevus of large spindle and/or epithelioid cells” reflects the characteristic cytologic appearances that provide the essential definition for the lesion (185). Because of the large size of the lesional cells often with considerable nuclear and cytoplasmic pleomorphism, and the frequent presence of an inflammatory infiltrate, the histologic picture often resembles that of a nodular melanoma. There is no doubt that, before the recognition of Spitz nevi as an entity, many cases were diagnosed as melanoma. Even today, differentiation from a melanoma can often be very difficult and occasionally even impossible. Features that aid in the distinction may be summarized as architectural pattern and cytologic features (Fig. 28-10B–G).
In terms of their fundamental architectural pattern, Spitz nevi resemble common nevi. They are usually compound, but they can be entirely dermal or junctional. They are small, symmetrical, and well-circumscribed. It is somewhat unusual for the intraepidermal component to extend beyond the dermal component, and such a finding should prompt consideration of associated dysplasia or melanoma. The epidermal component is arranged in nests often formed by spindle cells that tend to be oriented vertically and, though large, do not show significant variation in size and shape or tend to become confluent. The cytoarchitecture of spindle cells aligned in parallel within the junctional nests is characteristic, and forms the basis for the descriptive terms of nests that are considered to be “raining down” or that resemble “bunches of bananas.” In Spitz nevi with junctional activity, there are often artifactual semilunar clefts separating nests of nevus cells at the epidermal–dermal junction from adjacent and overlying epidermal cells. Not so commonly seen in melanoma, this feature represents a useful diagnostic feature.
Although there may be diffuse junctional activity, permeation of the epidermis by tumor cells (pagetoid melanocytosis) is relatively slight. If present, it usually consists of single nevus cells or small groups of cells and is generally limited to the lower half of the epidermis (Fig. 28-10G) (185). In a few Spitz nevi, however, pagetoid migration of lesional cells into the epidermis may be quite marked, especially in young children. These “pagetoid Spitz nevi” appear clinically as a small (<0.4 cm) pigmented macule in young patients. Features favoring nevus over melanoma include small size, circumscription, symmetry, even distribution of cells, and lack of marked cytologic atypia (195). Pagetoid involvement of the suprabasal epidermis is not a common feature of Spitz nevi in adults; in such a case, the diagnosis of melanoma should be considered (196). Occasionally, nests of lesional cells are seen in transit through the epidermis, described as transepidermal elimination of nevus cells (197,198).
The epidermis involved by Spitz nevi is often hyperplasic with elongated rete ridges. Occasionally, this hyperplasia is sufficiently florid to be termed pseudoepitheliomatous, representing a possible source of confusion with squamous cell carcinoma especially in a superficial biopsy (199). However, the epidermis may be thinned and even ulcerated, especially in very young children. A pattern termed “consumption of the epidermis,” defined as “thinning of the epidermis with attenuation of the basal and suprabasal layers and loss of rete ridges in areas of direct contact with neoplastic melanocytes” has been suggested as an additional criterion for differentiating melanoma from nevi. This feature is seen in many melanomas but is absent in most Spitz tumors (200). Found in fewer than half of the patients, diffuse edema, stromal hyalinization, and telangiectasia in the papillary dermis, if present, are of slight diagnostic importance. The edema may cause a loose arrangement of the nevus cell nests.
A useful though not pathognomonic cytologic criterion for Spitz nevi is the presence within the epidermis of dull pink globules resembling colloid bodies in 60% to 80% of cases. They may form larger bodies through coalescence. These “Kamino bodies,” are most commonly seen in the basal layer above the tips of dermal papillae (Fig. 28-10E). Similar-appearing eosinophilic globules have been noted in the epidermis in only 2% of melanomas and 0.9% of ordinary nevi, in which they are, however, less conspicuous because they do not coalesce (201,202). Although often these bodies may resemble apoptotic cells, formal studies have found no evidence of active apoptosis, and have related the eosinophilic material to hyalinized collagen or basement membrane–like material (203).
Important cytologic features of Spitz nevi include especially the presence of large spindle cells and epithelioid cells (Fig. 28-10C). Spindle cells or epithelioid cells may predominate, or the two types of cells may be intermingled (“large spindle and/or epithelioid cells”) (185). Apart from the shape of their cell bodies, the spindle cells and the epithelioid cells in any given Spitz nevus resemble one another in nuclear and in cytoplasmic texture, suggesting that they may represent dimorphic expression of a single cell type. The cells have abundant amphophilic cytoplasm that may contain scant, finely divided melanin pigment, although in most of the cells, pigment is typically absent. Upon close inspection, the cytoplasm may sometimes appear finely and faintly vacuolated, or show an amphophilic net-like character. The nuclei are large, with pale, delicate, and evenly dispersed chromatin and regular, smooth uniform, and delicate nuclear membranes, and prominent eosinophilic or amphophilic nucleoli. The size of the lesional cells, more than any other feature, sets the Spitz nevus apart from the common nevus (185), and also from most melanomas. Bizarre giant cells may be seen both in melanoma and Spitz nevi, the difference being that, in the latter, they usually have regular nuclei of similar size, whereas in melanoma the nuclei are usually more pleomorphic.
Mitoses are absent in about half of the cases. This is helpful in ruling out melanoma in these lesions. Usually, there are only a few mitoses, but occasionally, they are quite numerous in the epidermal compartment. A dermal mitotic rate of greater than 2 mitoses per mm2, abnormal mitoses, or mitoses close to the base of the dermal component (within the lower half of the dermal component) are unusual in Spitz nevi (204) and may warrant a diagnosis of melanoma or a descriptive diagnosis of “melanocytic tumor of uncertain potential.” Atypical mitoses are uncommon, and, if they are found, the lesion should be interpreted with great caution (204,205). The complete absence of mitoses in 50% of Spitz nevi is very helpful in ruling out melanoma in these cases.
Of special importance is maturation of the cells with increasing depth, so that they become smaller and look more like the cells of a common nevus (Fig. 28-10D–F). Also important is the uniformity of the lesional cells from one side of the lesion to the other: at any given level of the lesion from the epidermis to its base, the lesional cells look the same. Although large superficially, the cells at the base of most Spitz nevi are small, and they tend to disperse as single cells or files of single cells among reticular dermis collagen bundles. Involvement of the reticular dermis is highly characteristic of Spitz nevi, and as they descend into this part of the dermis, the cells become dispersed as single cells and some of them become separated from the apparent border of the lesion to form “outlier cells” (191) that can be revealed, for example, by a stain for S100 antigen. Other benign nevi also tend to infiltrate in this way if they involve the reticular dermis (206). Melanomas, in contrast, tend to form solid tongues or fascicles of tumor cells that separate and displace the collagen bundles in the reticular dermis, without forming outlier cells.
Melanin is in many instances completely or nearly absent in Spitz nevi. In a few cases, melanin is moderate or dense. Some of these “pigmented Spitz nevi” are better classified as pigmented spindle cell nevi, related lesions that are discussed in the next section. An inflammatory infiltrate is found in many Spitz nevi and may be quite heavy. Its distribution can be band-like, mainly at the base, as in some melanomas. Often, however, the infiltrate is patchy around blood vessels and is seen throughout the lesion (191,205,207).
Desmoplastic Spitz Nevi. In some examples of Spitz nevi (and also in some nevi with smaller cells that may not meet criteria for Spitz nevi), diffuse fibrosis is present (208–210). These desmoplastic Spitz nevi generally show no junctional activity, nesting, or pigmentation. The nevus cells are predominantly spindle-shaped and compressed by a desmoplastic stroma. However, they differ from a dermatofibroma by the presence of epithelioid and often also multinucleated cells. Desmoplastic melanoma (p. 920) almost always shows an associated lentiginous in situ component, in contrast to the rarity of junctional activity in desmoplastic Spitz nevi. Furthermore, desmoplastic melanomas are almost invariably negative (in their spindle cell component) for the HMB-45 and Melan-A (MART-1) melanocytic markers, which in contrast are usually positive in Spitz nevi (211,212). Some desmoplastic nevi have clusters of lymphocytes, a finding that can raise concern for desmoplastic melanoma; however, in a study of six such lesions, other features of melanoma were lacking and the lesions all expressed Melan-A and the tumor suppressor p16, and had negative results by FISH testing for melanoma (213). In another study, all of 5 desmoplastic nevi strongly expressed for p16; however, 6 of 22 desmoplastic melanomas were also diffusely positive, indicating that this is not a reliable marker for this distinction (194,214).
Hyalinizing Spitz Nevi. These are related lesions that present with spindle or epithelioid nevus cells embedded in a paucicellular hyalinized collagenous stroma. Some of these lesions have been mistaken histologically for metastatic carcinomas (215).
Angiomatoid Spitz Nevi. These lesions present histologically with large spindle and/or epithelioid cells placed among “angiomatoid” densely arranged, small blood vessels lined by plump endothelial cells embedded in a collagenous stroma. The Spitz nevus cells may be quite inconspicuous among the vessels in some cases. There have been no reported cases of recurrence or metastases (216).
Spitz Nevus of Special Sites. Occasionally, a Spitz nevus will involve a special site (e.g., acral). In such cases, it is important to recognize the retention of the basic architectural and cytologic features of a Spitz nevus (as described above), albeit in the context of potentially confounding site-dependent architectural characteristics. The ability to define such lesions as Spitz nevi from the outset is critical to avoiding overdiagnosis where the perceived degree of cytologic atypia in the context of the site may lead to an erroneous diagnosis of melanoma.
Histogenesis. On electron microscopic examination, Spitz nevi show in their upper portion melanocytes with numerous melanosomes; in their lower portion, the number of melanosomes in the melanocytes decreases. Melanization is incomplete in most melanosomes, and there is evidence of lysosomal degradation of melanosome complexes (217). The paucity of melanin in most Spitz nevi is thus explained. Biological marker studies have shed little or no light on the mechanisms whereby Spitz nevi appear to have the capacities for relatively rapid invasive and tumorigenic proliferation in the dermis, but to lack the capacity for metastasis.
The genetic basis of Spitz tumors is now beginning to be understood, and there are important differences compared to common nevi and to melanomas. In a seminal study by Bastian et al. using comparative genomic hybridization and FISH), copy number increases often associated with oncogenic mutations involving the HRAS gene on chromosome 11p were found in about 10% of cases of Spitz tumors. The tumors with 11p copy number increases were larger, predominantly intradermal, had marked desmoplasia, characteristic cytologic features, and had an infiltrating growth pattern. HRAS activation by either mutation or copy number increase alone could explain several of the histologic features that overlap with those of melanoma. It is speculated that HRAS activation in the absence of cooperating additional genetic alterations may drive the partially transformed melanocytes of these “atypical” Spitz nevi into senescence or a stable growth arrest (218), likely as a result of p16 activation in a process termed “oncogene-induced senescence” (219). There are no data to suggest that Spitz nevi with HRAS activation are at special risk for progression to melanoma; however, the biological potential of these lesions is not completely understood.
A subset of Spitzoid tumors has recently been found to be associated with loss of the tumor suppressor, BAP1, which is also associated with ocular melanoma. In a study of 32 atypical Spitz tumors (AST) for BRAF mutations and for BAP1 expression, 9 showed loss of BAP1 expression, of which 8 had concomitant BRAF mutations (which are rare in most other Spitz tumors). The BRAF-mutated, BAP1-negative tumors were primarily dermal and were composed predominantly of epithelioid melanocytes with abundant amphophilic cytoplasm and well-defined cytoplasmic borders. Nuclei were commonly vesicular and exhibited substantial pleomorphism and conspicuous nucleoli (220). The lesions are commonly situated within a congenital pattern nevus in which BAP-1 expression is preserved, constituting a type of combined nevus. To date, the biological behavior of these lesions has been benign.
In general, with the exception of the few HRAS- and BRAF-mutated Spitz tumor already mentioned, mutations of traditional oncogenes have been rarely demonstrated in these tumors. In a recent study by Bastian’s group, 20 benign Spitz nevi and 8 AST, which had been selected for morphologic features inconsistent with HRAS or BRAF/BAP1 mutations, were screened for mutations of known cancer-causing genes and for kinase fusion genes. Only a single case had a point mutation in HRAS, and no known alterations were found in other known oncogenes. Remarkably, genomic rearrangements were observed in 19 cases. These rearrangements fused the intact kinase domains of ROS1 (36%), ALK (14%), RET (7%), NTRK1 (7%), and BRAF (4%) to a wide range of predominantly novel 5’ partners, forming constitutively activated chimeric oncogenes. The fusions occurred in a mutually exclusive pattern and were more common in younger patients. These gene fusions found in two-thirds of Spitz tumors are likely to be useful as diagnostic markers and as potential therapeutic targets for those few of these tumors that may metastasize (221).
Diagnostic and Prognostic Markers. Morphometric and immunohistochemical studies have shown differences between Spitz nevi and melanomas, but none of these adjuncts to histologic diagnosis is in standard use. By immunohistochemistry, Spitz nevi react not only with differentiation markers for melanocytes such as S100 antigen, but also with HMB-45 and Melan-A/MART-1, the former a melanosomal antigen often expressed in proliferating melanocytic lesions (as well as melanoma), and the latter a more specific marker of melanocytic lineage (134,211,212). Spitz nevi tend to show a more orderly “top heavy” pattern of stratification of HMB-45 reactivity from superficial to deep compared to melanomas (211). A recent study demonstrated interesting differences in S100A6 protein expression between Spitz nevi and melanomas (222). Like nevi and unlike melanomas, Spitz nevi show a tendency toward diminution of nuclear size and/or DNA content (223,224), as well as for reactivity with certain antigens, from the superficial to the deep portions of the nevus (211). This stratification may be regarded as an expression of maturation/senescence, which is diagnostically and no doubt also biologically important in the distinction between Spitz nevi and melanomas. In a study of S-100A6, HMB-45, and MIB-1/Ki-67 in 121 Spitzoid tumors, this panel of immunostains made an independent contribution to the final diagnosis; the magnitude of this contribution was considered to be similar to that of considering the findings in hematoxylin- and eosin-stained sections together with the patient’s age (225).
Cell proliferation and cell cycle markers are important discriminators between benign, atypical, and malignant spitzoid neoplasms. The simplest of these is the determination of mitotic rate. Studies by Crotty and colleagues demonstrated that the presence of abnormal mitoses, a dermal mitotic rate of greater than 2/mm2, and mitotic figures within 0.25 mm of the deep border of the lesion favored a melanoma diagnosis (204). Using the proliferation marker Ki-67/Mib-1, the same group had previously found that the maximal (i.e., “hot spot) number of positive nuclei was about 10 in the Spitz tumors versus 32 in the melanomas studied, indicating quite good discrimination (226). Vollmer has emphasized the important interactions between age and proliferative activity, indicating from a meta-analysis of the literature that a Ki-67 proliferation rate of more than 10% favors a melanoma diagnosis and less than 2%, a Spitz nevus. Values between 2% and 10% yield various predictive values for Spitz nevus, depending on the a priori probability of this diagnosis based on age and other factors (i.e., clinical, histologic, and marker studies) (227). In general, melanoma is very rare in prepubertal children, and especially under the age of 2 years.
The cell cycle and tumor suppression molecule p16 appears to be an important marker for benign Spitz nevi and for nevi in general. In a study of p16 expression in 6 Spitzoid malignant melanomas, compared to 18 Spitz nevi, and 12 compound nevi in children younger than 18 years, all of the childhood melanoma cases were associated with loss of p16, whereas all the Spitz and other benign nevi had strong positive nuclear and cytoplasmic expression of p16, suggesting that this marker should be of considerable value for this distinction (228).
In the last few years, comparative genomic hybridization (CGH) and FISH have been applied to series of cases of Spitzoid and other neoplasms. In a CGH study, a limited variety of copy number aberrations including gains of 11p and much more rarely 7q were seen in Spitz nevi. Conversely, melanomas with spitzoid features typically have multiple chromosomal copy number aberrations involving a variety of loci (229). Markers derived from CGH were used in a large study of 64 AST with 5 years of uneventful follow-up, and 11 AST that had resulted in advanced locoregional disease, distant metastasis, or death. Gains in 6p25 or 11q13 and homozygous deletions in 9p21 had statistically significant association with aggressive clinical behavior with P-values of .02, .02, and <.0001, respectively. In multivariate analysis, homozygous 9p21 deletion was highly associated with clinically aggressive behavior (P < .0001) and death due to disease (P = .003) (230). In another study, of 31 Spitzoid lesions, 9p21 loss was present in the only fatal case of a Spitzoid melanoma (in a 41-year-old man), but 5 cases with 9p21 deletions by FISH were alive and well at follow-up intervals ranging from 6 to 135 months, indicating that 9p21 loss alone does not predict a fatal outcome (231).
The chromosomal locus 9p21 includes the site of the tumor suppressor gene p16, which is downregulated in essentially all melanomas by one means or another, including homozygous loss as demonstrated in the FISH study reviewed above. In that study, increased mitotic rate and homozygous 9p21 loss were the only independent factors predicting mortality from spitzoid lesions. This finding reinforces the potential value of p16 immunostaining in assessing spitzoid tumors. If a p16 immunostain is positive, then 9p21 loss cannot have occurred, favoring a benign prognosis. If the p16 stain is negative, then FISH or CGH could be done, and if homozygous 9p21 loss is identified, the evidence suggests that management of the lesion as having the potential for systemic metastasis would be appropriate, while recognizing that the prognosis of spitzoid melanomas is better than that for usual melanomas, and 9p21 loss alone does not predict a fatal outcome (231).
Differential Diagnosis. The distinction between Spitz nevi/tumors and benign nevi is usually trivial except for dysplastic nevi, which may be indicators of an individual at increased risk for melanoma. In cases of doubt in this regard, we add a note to the effect that the differential diagnosis may include a dysplastic nevus, and recommend that the patient be evaluated for other risk factors, such as multiple dysplastic nevi, and a family or personal history of melanoma. Management can then be based on the patient’s overall risk profile.
Occasional examples of histiocytic tumors such as epithelioid histiocytomas or juvenile xanthogranulomas may be confused with epithelioid Spitz nevi or with melanomas, especially when foam cells or Touton giant cells are inconspicuous (232). It has been demonstrated that Melan-A and tyrosinase markers are sensitive and specific in making the diagnosis of a melanocytic lesion, while histiocytic markers such as CD68 and Factor XIIIa are likely to be positive in the histiocytic lesions (see Chapter 26) (233–235).
The differential diagnosis with melanoma is of course of much greater importance. Differentiation of a Spitz nevus from a nodular melanoma can be difficult and even impossible in some cases, because all of the changes seen in the Spitz nevus may also be observed in melanoma. This differential has been discussed extensively above. Crotty and colleagues consider that that the presence of symmetry, Kamino bodies, and uniformity of cell nests or sheets from side-to-side favor a Spitz nevus, while the presence of abnormal mitoses, a dermal mitotic rate of greater than 2/mm2, and mitotic figures within 0.25 mm of the deep border of the lesion favor a melanoma (204). To this list we would add the characteristic dispersion pattern of single cells at the base, which favors a Spitz or other benign nevus (Table 28-3).
Atypical Spitz Tumor and Spitzoid Melanoma
Spatz and Barnhill have provided a set of criteria for grading risk in AST considering metastasizing cases in childhood. These criteria included diagnosis at age greater than 10 years, diameter of the lesion greater than 10 mm, presence of ulceration, involvement of the subcutaneous fat (level V), and mitotic activity of at least 6/mm2 (188). As noted above, it is not always possible to make an unequivocal distinction between a melanoma or a Spitz tumor/nevus, and in these doubtful cases we will often use the descriptive term “Melanocytic (or Spitzoid) tumor of uncertain potential” (MELTUMP or STUMP), and provide a differential diagnosis, which might in cases of extreme doubt include microstaging attributes that would apply if the lesion were interpreted as a melanoma (236). Cerroni et al. studied 57 cases of MELTUMP with follow-up of at least 5 years. The only three histopathologic criteria that were statistically different between the groups of favorable and unfavorable cases were presence of mitoses, mitoses near the base, and an inflammatory reaction. It was considered that these lesions as a group exist and that they may be biologically different from conventional melanoma and benign melanocytic nevi. The controversial terminology reflects the uncertainty in classification and interpretation of these atypical melanocytic tumors (237).
Genomic testing using FISH has the potential to identify those few cases for which more aggressive management may be appropriate to consider. In a series of 75 AST collected from several institutions, all of the tumors had one or more chromosomal copy number aberrations. The presence of homozygous 9p21 deletions and a positive sentinel lymph node (SLN) were each found to be correlated with tumor extension beyond the SLN. Two patients with ASTs that had homozygous 9p21 deletions developed brain metastasis, one of whom died of disease (238). The 9p21 locus contains the p16 tumor suppressor gene, suggesting that a rational first approach to an AST may be an immunohistochemical stain for p16, as discussed above (p. 883). If this is negative, consideration of FISH and/or CGH studies would be supported. A tumor that has “atypical” attributes as mentioned above, and has homozygous loss of 9P21, could reasonably be considered as a “Spitzoid melanoma.” Nevertheless, especially in young children, progressive disease resulting in death is vanishingly rare in Spitzoid neoplasms.
Approaches to Management of Spitz Tumors
Because of the difficulty of making an absolutely certain differentiation from melanoma, it is advisable as a precautionary measure in our opinion that lesions diagnosed as Spitz tumors/nevi be excised completely, especially in persons at or beyond puberty, particularly since such lesions are usually small in size. Although the issue is debatable (239), exceptions to this general rule might include those lesions where there are cosmetic or other contraindications to excision, and where the diagnosis of a benign Spitz nevus is certain despite the partial nature of the biopsy (240). Also, one should never exclude a diagnosis of melanoma based on age alone, because melanomas may arise in children, although this is very rare (p. 928).
There are cases on record that were diagnosed as instances of Spitz nevus but later proved to be lethal melanomas, while other cases have metastasized to regional lymph nodes but not beyond (241,242). These lesions, often large, ulcerated, deeply infiltrative, and mitotically active, can alternatively be signed out descriptively as “melanocytic tumors of uncertain malignant potential” (p. 930). Others regard these lesions as “Spitzoid melanomas,” with most considering that the prognosis for these tumors may be better than expected for an ordinary melanoma with similar microstaging attributes (187). The diagnosis of a Spitz nevus/tumor depends on an assessment of multiple morphologic features, which are summarized above and in Table 28-3, p. 884. Given that so-called metastasizing Spitz nevi/tumors are usually associated with at least medium- to long-term survival (243–247), it may be appropriate to regard the metastases as “metastatic melanocytic tumors of uncertain potential,” rather than unqualified metastatic malignant melanoma. However, in most institutions, such individuals would be offered a consideration of adjuvant protocols for metastatic melanoma.
Sentinel node staging has been considered for AST and Spitzoid melanomas. A positive sentinel node has been found in approximately 30% of cases in several published series of cases (243–248). However, the incidence of progressive disease and death is very low, especially in children and young adults. Therefore, sentinel node staging cannot be considered standard of care for these lesions (249).
Pigmented Spindle Cell Nevus
This tumor, first described by Richard Reed in 1973 (250), may be regarded as a variant of the Spitz nevus (177,185), or as a distinctive clinicopathologic entity (251). In our experience and that of others, most cases differ significantly from classical Spitz nevi, but some present with overlapping features, indicative of a close relationship between the two entities (252–254). Although this distinction has no clinical significance in that each is a benign lesion, it is important to rule out melanoma, and this is facilitated by an understanding of the differences between these two common melanoma simulants.
Clinical Summary. The lesions are usually 3 to 6 mm in diameter, deeply pigmented, and either flat or slightly raised. Most patients are young adults, and the most common location is on the lower extremities. Pigmented spindle cell nevi are uncommon after the age of 35. A classical presentation is that of a newly evolved black plaque on the thigh of a young woman. Because of the heavy pigment and the history of sudden appearance, a diagnosis of melanoma is often suspected clinically. In contrast, Spitz nevi are usually submitted with a benign clinical diagnosis, such as an angioma or a dermal nevus. Like Spitz nevi, the lesions are generally stable after a relatively sudden appearance and a short-lived period of growth.
Histopathology. The pigmented spindle cell nevus is characterized by its relatively small size and its symmetry, and by a proliferation of uniform, narrow elongated spindle-shaped, often heavily pigmented melanocytes at the dermal–epidermal junction (251–253,255). The nests of spindle cells are vertically oriented, and tend to blend with adjacent keratinocytes rather than forming clefts as in Spitz nevi. Eosinophilic globules (“Kamino bodies”) may be present (256) (Fig. 28-11). The tumor cells often form bundles that are separated by elongated rete ridges. In the papillary dermis, the nevus cells lie in compact clusters, pushing the connective tissue aside. Numerous melanophages are characteristically diffusely present within the underlying papillary dermis. Involvement of the reticular dermis, common in Spitz nevi, is unusual in pigmented spindle cell nevus. Some lesions show upward epidermal extension of junctional nests of melanocytes. Single-cell upward invasion of the epidermis in a pagetoid pattern may be present but is usually not prominent (163,252). Hypopigmented examples have been described, having all the typical features of conventional pigmented spindle cell nevus, but not containing abundant melanin (257).
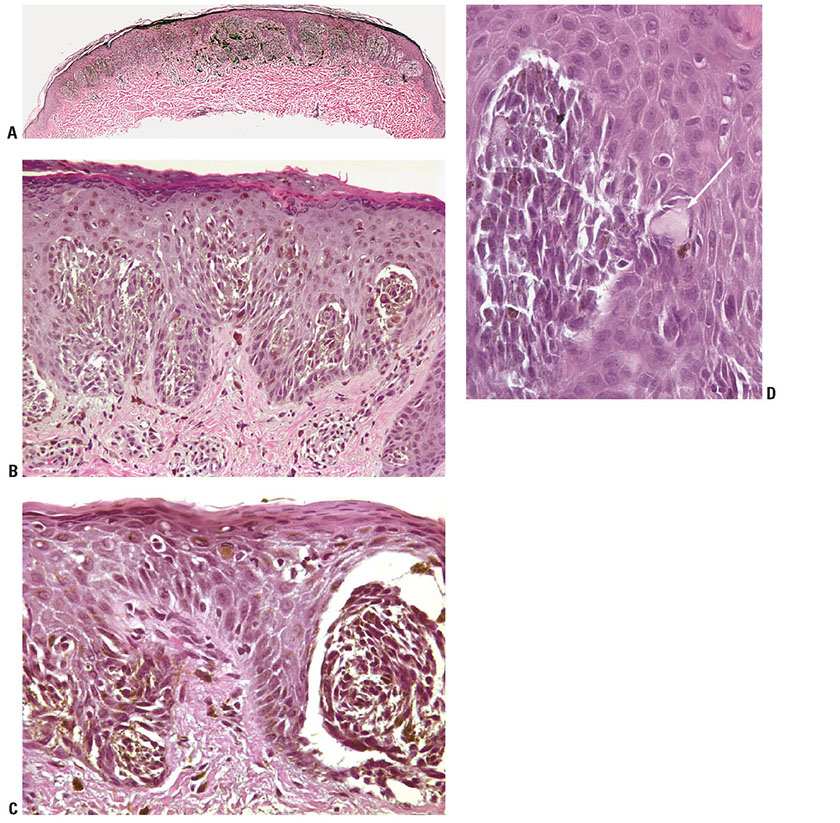
Figure 28-11 A: Pigmented spindle cell nevus. The typical configuration of this lesion is that of a small plaque whose breadth is considerably greater than its height. The lesional cells are typically junctional or confined to the epidermis and the papillary dermis. B: As in the classic Spitz nevus, the lesional cells are arranged in nests that tend to be vertically oriented. Unlike those of the Spitz nevus, the cells are narrow elongated spindle cells without epithelioid cells, and they contain abundant usually coarse melanin pigment. C: As in Spitz nevi, some (usually slight) degree of pagetoid melanocytosis is not unexpected. Mitoses may be numerous in the epidermis, but the lesional cells in the dermis tend to be mature, with few, if any, mitoses. Clefting artifact between the nests and the adjacent keratinocytes tends to be less prominent than in Spitz nevi, but may be present, as here. D: Kamino body in a pigmented spindle cell nevus. This nevus has a prominent eosinophilic globoid “Kamino body” (arrow) in its epidermal compartment.
Features that may lead to a diagnosis qualified as “atypical pigmented spindle cell nevus” include architectural abnormalities including poor circumscription and pagetoid melanocytosis, prominent cytologic atypia, or a prominent epithelioid cell component (252). There may also be considerable overlap with dysplastic nevi (252). The significance of these “atypical” variants appears to lie in their greater chance of being misdiagnosed as melanoma, since all reports of pigmented spindle cell nevi emphasize their benign behavior after excision.
Differential Diagnosis. The most important differential is with melanoma of the superficial spreading type. In contrast to these melanomas, pigmented spindle cell nevi are smaller, symmetrical, and show sharply demarcated lateral margins. The tumor cells appear strikingly uniform “from side to side.” If lesional cells of pigmented spindle cell nevi descend into the papillary dermis, they mature along nevus lines, in contrast to melanomas. Mitoses may be present in the epidermis in either lesion, but are uncommon in the dermis in pigmented spindle cell nevi. Abnormal mitoses are exceedingly uncommon. Pagetoid melanocytosis is usually not prominent, and the spindle cell cytology differs from that of the epithelioid cells that predominate in most superficial spreading melanomas (SSMs). Lentigo maligna melanomas (LMMs) may have spindle cells in their vertical growth phase (VGP) component, but the epidermal component is usually composed of smaller, nevoid albeit atypical cells. Some pigmented spindle cell nevi may present overlapping features with dysplastic nevi, but can usually be distinguished on the basis of their irregularly thickened epidermis, their vertically oriented nests, and the uniformity of cell type, the absence of cytologic dysplasia, and the absence of typical stromal alterations (e.g., lamellar fibrosis). In cases of extreme doubt, we may classify the lesions as “superficial atypical melanocytic proliferation” (SAMPUS) and provide a differential diagnosis of melanoma in situ or dysplastic nevus. In the former case, a reexcision procedure would be recommended, while in the latter case we would recommend assessment of melanoma risk factors and perhaps follow-up, especially if the patients have other clinically atypical nevi or a family or personal history of melanoma (236). Some rare examples of lesions with larger expansile intradermal nodules may be difficult to distinguish from melanoma and a descriptive diagnosis may be appropriate (“melanocytic tumor of uncertain potential,” MELTUMP, p. 930).
Principles of Management. Pigmented spindle cell nevi and related lesions generally do not require active therapy. Lesions are often excised for cosmetic reasons, and to rule out melanoma. The presence of such a lesion is not a risk factor for future development of melanoma.
Congenital Melanocytic Nevus
A congenital melanocytic nevus (CMN) may be defined as a lesion present at birth and containing nevus cells.
Clinical Summary. A congenital melanocytic nevus may be defined as a lesion present at birth and containing nevus cells. Congenital nevi are found in about 1% to 2% of newborn infants (258,259), and thus, although such lesions are rare when compared to the total number of all non-congenital nevi, their occurrence is a relatively common clinical event. In many instances, congenital nevi are larger than acquired nevi, measuring more than 1.5 cm in diameter. However, only a few are of considerable size. Those measuring more than 20 cm in greatest diameter are referred to as giant CMN (Fig. 28-12A) (260). Non-giant CMN are usually slightly raised and often pigmented, and they may show a moderate growth of hair. They may be classified as “small” (<1.5 cm in diameter) or “intermediate” (>1.5 cm and <20 cm, or amenable to local excision) (260,261). A number of variants have been described, such as the cerebriform congenital nevus, which presents as a skin-colored, convoluted mass (262); the spotted grouped pigmented nevi, showing closely set brown to black papules (263); the congenital acral melanocytic nevus, which consists of a blue black patch on the sole or the distal portion of a finger clinically resembling an acral-lentiginous melanoma (264); and the desmoplastic hairless hypopigmented nevus, which presents clinically as a hard, ligneous, progressively hypopigmented and alopecic giant congenital nevus and is characterized histologically by intense dermal fibrosis, scarce nevus cells, and hypotrophic or absent hair follicles (265). Giant CMN often have the distribution of a garment (“garment nevi”). They usually are deeply pigmented and are covered with a moderate growth of hair. Often, there are many scattered “satellite” lesions of a similar appearance (266). These satellite nevi are benign, in contrast to the satellite metastases that may be associated with melanomas. Leptomeningeal melanocytosis may be present especially in cases in which the giant congenital nevus involves the neck and scalp. There may be not only epilepsy and mental retardation but a primary leptomeningeal melanoma may also occur (267). In one study, magnetic resonance findings of meningeal melanosis were identified in 14 of 43 asymptomatic children with giant CMN. These findings are consistent with an increased lifetime risk of central nervous system melanoma (268).
Figure 28-12 A: Giant congenital nevus (clinical). These lesions have also been called “garment nevi” because of their coverage of such large areas of skin. Focal areas of increased pigmentation, sometimes associated with palpable nodularity, are not uncommon in giant congenital nevi. Such foci should be followed carefully, and any lesions showing evidence of progressive changes should be excised to rule out melanoma. B: Melanoma in a giant congenital nevus. This nodule developed on the back of the patient illustrated in A, at the age of 4 years. The patient died within about 2 years of metastatic melanoma.
Incidence of Cutaneous Melanoma. In a comprehensive meta-analysis, 14 articles were chosen from a large literature for further analysis. The frequency of melanomas ranged between 0.05% and 10.7% and was significantly higher in smaller studies. In a total of 6,571 patients with congenital nevi who were followed for a mean of 3.4 to 23.7 years, 46 patients (0.7%) developed 49 melanomas, a lower number than might have been expected, probably reflecting selection bias in published smaller studies. The mean age at diagnosis of melanoma was 15.5 years (median 7), indicative of a maximum risk in childhood and adolescence. By comparison with age-adjusted population-based data, the patients with congenital nevi carry an approximately 465-fold increased relative risk of developing melanoma during childhood and adolescence. Primary melanomas arose inside the nevi in 33 of 49 cases (67%). In seven cases (14%), metastatic melanoma with unknown primary was encountered; in four cases (8%), the melanoma developed at an extracutaneous site (269).
Of note, the majority of patients with congenital nevi, even large ones, will never develop melanoma. In one study, increasing numbers of satellite lesions and larger lesional diameters were associated with melanoma and neurocutaneous melanosis (270). The melanoma may be present at birth, or it may arise in infancy or any time later in life (Fig. 28-12B). The mortality of such melanomas is substantial. In a cohort study in which 33 patients with giant congenital nevi were followed, 2 fatal melanomas developed, representing a relative risk approximately 1,000-fold greater than that in the general population (271), while in another study of 80 pediatric cases, 4 melanomas (3 of them fatal) occurred after an average follow-up of about 5 years (272). In follow-up data on 170 patients in the New York University Registry of Large Congenital Melanocytic Nevi, 4 melanomas had developed, including extracutaneous melanomas in the central nervous system and 1 retroperitoneal melanoma (270,271,273). It is therefore generally agreed that it would be desirable for giant melanocytic nevi to be excised, if feasible. However, complete excision is often not possible, and melanomas may develop in extracutaneous sites. Thus, clinical surveillance is considered to be an acceptable alternative in such situations (273,274).
The incidence of melanoma in non-giant congenital nevi, those less than 20 cm in greatest diameter, is unknown, but probably greater than that in a comparable area of normal skin. It is likely that the risk is related to the lesion’s size (275).
Histopathology. The histologic appearance of giant congenital nevi differs from that of acquired nevi in terms of their greater size and depth, and in the involvement of skin appendages and sometimes even deeper tissues (e.g., galea aponeurotica and cranial nerve extension in scalp lesions) (Fig. 28-13). Non-giant congenital nevi may have the same histologic appearance as acquired nevi, or may show features of congenital nevi (Fig. 28-14) (123,276,277). The features in non-giant congenital nevi are neither sensitive nor specific for truly congenital origin of a nevus: a recent histologic study with comparison with clinical data showed that 32 nevi with the histologic criteria of congenital nevi were actually acquired, and that 179 nevi present at birth did not fulfill these criteria. However, such studies are complicated by the possibility that some congenital nevi may be clinically covert at birth, and thus regarded as acquired. In the case of deep and large (giant) congenital nevi, in contrast, the clinicopathologic correlation was 100% (278). Cytologically, the cells of congenital nevi are similar to those of acquired nevi. The nevus cells are typically positive for the melanocytic markers S100, HMB-45, and Melan-A, the last of which has the best combination of sensitivity and specificity (134).
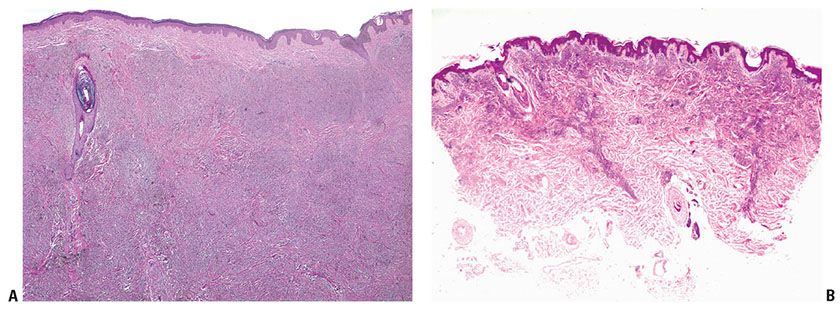
Figure 28-13 Giant congenital nevus. A: This nevus is very broad (many centimeters, extending well beyond the borders of the image), and “deep”, that is, the lesional cells extend from the epidermis through the reticular dermis and into the fat. B: In another giant congenital nevus, sheets of nevus cells are placed among reticular dermis collagen bundles. The dermal component of giant congenital nevi is often variably cellular, and in addition to nevic differentiation, there may be evidence of Schwannian or even heterotopic elements.
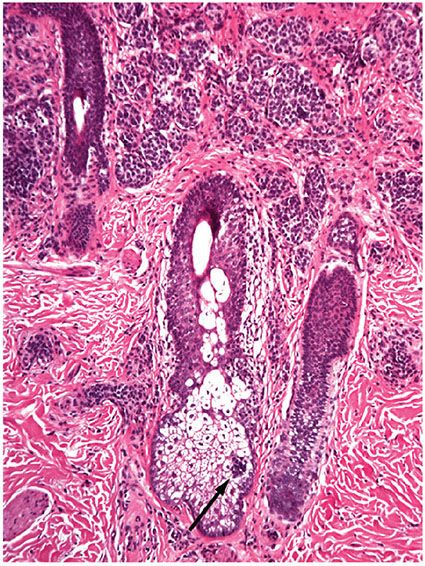
Figure 28-14 Compound nevus with congenital pattern features. Nevus cells extend around skin appendages and between fibers of the reticular dermis. The presence of nevus cells “within” skin appendages (as here in the sebaceous unit) is a quite specific feature indicating true congenital onset of a nevus. The extension of nevus cells into the upper reticular dermis and around skin appendages is quite characteristic of congenital nevi. However, acquired nevi may also exhibit this “deep” pattern, and conversely, congenital nevi are not always “deep.”
Non-Giant (Small and Intermediate) CMN. At one time these were thought to be superficial at birth and show deep involvement later. However, a serial study showed no change in pattern in follow-up biopsies (279). This and other studies have shown various patterns in the distribution of the nevus cells independent of the age of the patient. Thus, non-giant congenital nevi may be junctional, compound, or intradermal nevi, and their location in the dermis may be either superficial, which may include junctional involvement, or superficial and deep (279,280). They may differ from acquired nevi by one or more features: (a) presence of melanocytes, often in nests, around and within hair follicles, in sweat ducts and glands, in sebaceous glands, in or in intimate association with vessel walls, in arrector pili muscles, and in the perineurium of nerves; (b) extension of melanocytes between collagen bundles singly or in double rows; and (c) extension into the deepest reticular dermis and into the subcutis (Fig. 28-14) (281). However, many non-giant congenital nevi show none of these features. For example, some documented congenital nevi have been entirely junctional (279), while the likelihood of deep dermal involvement appears to increase with the size of the lesion (282) and is actually uncommon in lesions smaller than 3 cm (277). Conversely, the presence of most of the features mentioned above has been documented in nevi that were indubitably became clinically apparent after birth (and therefore called “tardive” congenital pattern nevi) (283). Thus, nevi with the constellation of features described above may be characterized as “nevi with congenital pattern features,” but not all of these lesions are truly congenital in origin. It appears that most congenital nevi have mutation of NRAS, in contrast to most acquired nevi that have mutated BRAF. The lesions appear to undergo oncogene-induced senescence, similar to acquired nevi (284). In another contrast, most of the “congenital pattern” nevi that are actually acquired have BRAF mutations, possibly related to sun exposure (285). It remains to be seen whether such nevi have any special significance as risk markers or potential precursors of melanoma (286).
Among the special forms of non-giant congenital nevi, the cerebriform congenital nevus usually presents as an intradermal nevus with neuroid changes simulating those seen in neurofibroma (262). Lesions called spotted grouped pigmented nevi also are intradermal nevi. They are either eccrine-centered or follicle-centered. If they are eccrine-centered, each eccrine sweat duct is tightly enveloped by nevus cells, whereas hair follicles are involved only slightly (263). If they are follicle-centered, nevus cell nests are found mainly around the hair follicles. In the acral nevus of congenital origin, a compound nevus is seen with considerable pigmentation in the upper dermis, and aggregates of nonpigmented nevus cells are seen around blood vessels and eccrine glands in the lower dermis (264). In a lesion called desmoplastic hairless hypopigmented nevus, there is intense dermal fibrosis, scarce nevus cells, and hypotrophic or absent hair follicles. Follow-up biopsies have documented the progressive nature of the fibrosis and nevus cell depletion (265).
In some CMN, changes are observed that may simulate a melanoma. In a recent study, features that could simulate malignant melanoma in a congenital nevus included: (a) asymmetry and poor circumscription, (b) an increased number of single melanocytes that predominated over nests of melanocytes in some high-power fields, (c) single melanocytes not equidistant from one another, (d) scattered single melanocytes present above the dermoepidermal junction, and (e) confluence of nests of melanocytes, all features in common with malignant melanoma (287). In our experience, such changes are generally slight in degree, and severe uniform cytologic atypia or frequent mitoses are not observed. Occasionally, a biopsy in a neonate may show disturbing cytologic and architectural atypia of junctional and/or dermal components; however, in our experience of several such cases, the lesions have subsequently undergone maturation and become indistinguishable from a benign congenital nevus. Therefore, a diagnosis of melanoma in a congenital nevus in a neonate should be made with great caution.
Giant CMN. Often these are more complex than nongiant congenital nevi. Three patterns may be found within them: a compound or intradermal nevus, a “neural nevus,” and a blue nevus pattern (267). In most instances, the compound or intradermal nevus component predominates, whereas, in others, the “neural nevus” component predominates. In the latter case, formations such as neuroid tubes and nevic corpuscles are present (p. 872). These areas may show considerable similarity to a neurofibroma. A component resembling a blue nevus or a CBN is found in some of the giant pigmented nevi, usually as a minor component. In rare instances, however, the entire congenital lesion consists of a giant blue nevus, which, in one patient with a lesion of the scalp was reported to extend to the dura (43) and in another had infiltrated the brain (44). Nevus cells may be present in lymph nodes draining skin containing a giant congenital nevus; this phenomenon should not be mistaken for involvement by a melanoma. In general, the nodal nevus cells lie in capsular or sinusoidal septal collagen, and the cells lack atypia (288).
Histogenesis. As is the case for common nevi, a dual origin of congenital nevi has been proposed and it seems likely that the dermal component of these lesions, at least, must be derived directly from neural crest precursors, rather than by a process of “abtropfung,” or “dropping down” from the epidermis. There is also evidence that these lesions differ genetically from common nevi. As in other nevi, oncogenic mutations in NRAS, BRAF, and Tp53 have been described in individual congenital nevus lesions. However, the pathogenesis of multiple CMN within the same subject has not been explained. Kinsler et al. found consistent oncogenic mutations in codon 61 of NRAS in affected neurologic and cutaneous tissues of 12 out of 15 patients, but not in normal tissues, consistent with NRAS mutation mosaicism. All 11 non-melanocytic and melanocytic CNS samples from 5 patients were mutation positive, despite NRAS rarely being reported as mutated in CNS tumors. These results suggest that single postzygotic NRAS mutations are responsible for multiple CMN and associated neurologic lesions in the majority of cases (289,290).
In studies of cellular differentiation in giant congenital nevi using melanocytic and stem cell markers, Kinsler et al. identified two groups of cases: those with nevus cell nesting and those with only diffuse dermal infiltration. Samples from the nested lesions were significantly more likely to express melanocytic differentiation markers, the expression of which decreased significantly with depth. The stem cell markers were often co-expressed. The dermal lesions frequently coexisted with normal overlying melanocyte development, suggesting that these lesions likely develop from a cutaneous stem cell independent of, or remaining after, normal melanocytic migration, and capable of some degree of melanocytic differentiation superficially (291).
Melanoma and Other Tumors in Congenital Nevi
A variety of benign and malignant tumors can occur in congenital nevi, the most important of which is malignant melanoma.
Clinical Summary. The pattern of occurrence of melanoma in giant congenital nevi was studied in a review of 34 patients who had primary cutaneous melanomas within their nevi; in 2 additional patients, melanoma developed at cutaneous sites other than within their nevi. All patients in whom melanoma developed within the nevi had nevi in axial locations; however, 91% of the nevi were axial. No melanoma was found that had arisen in any of the 26 nevi confined to the extremities. In addition, no melanoma was found that had arisen in thousands of satellite nevi (292). If a melanoma arises in a congenital nevus, it usually originates at the epidermal–dermal junction and the appearances are those of an ordinary form of melanoma, usually of the superficial spreading or nodular types (p. 904). Most of the congenital nevi associated with such melanomas are small and superficial (293). Occasionally, however, a melanoma in a giant congenital nevus, in contrast with nearly all other cutaneous melanomas, arises deep in the dermis or subcutis (294–297).
Histolopathology. In our experience, some of the melanomas in giant congenital nevi have consisted largely of undifferentiated “blastic” cells resembling lymphoblasts and containing little or no melanin, while others are comprised of large epithelioid cells similar to those of many melanomas. Other patterns of malignancy that have been described in congenital nevi have included neoplasms with the appearance of a neurosarcoma (298), lesions that have been termed malignant blue nevus (299–301), neoplasms with heterologous mesenchymal elements including rhabdomyoblasts (302) and lipoblasts, undifferentiated spindle cell cancers, and well-differentiated neoplasms termed minimal deviation melanomas (301). It has been emphasized that peculiar differentiation is to be expected in neoplasms of giant congenital nevi, and that alarmingly cellular neoplasms may not behave aggressively (301). Thus, pathology does not always readily predict outcome. This is especially true in our experience when melanomas arise in the first few months of life.
Cytologic and architectural atypia may occasionally be seen in congenital nevi, and criteria for the distinction of these lesions from melanoma are similar to those discussed in the differential diagnosis of dysplastic nevi and SSMs in later sections. In an interesting study, abnormal DNA content was found to correlate with cytologic atypia in giant congenital nevi (303). It is possible that the presence of atypical foci could increase the risk of malignant transformation.
Melanomas in small congenital nevi are typically of the usual superficial spreading type. In a recent study, 40 of 190 cases of melanoma were associated with preexisting nevi; of these, 15 had congenital features with a largest diameter of 1.5 cm, that is, “small congenital pattern nevi.” These 15 cases were melanomas of the superficial type with a mean tumor thickness lower than that of melanomas not associated with nevi (0.33 vs. 1.50). Thus, a relatively high percentage of small congenital pattern nevi was found to be associated with melanomas, indicating that they may be considered as potential melanoma precursors. Nevertheless, the risk of progression of any given lesion is very low (286).
Cellular and/or Proliferative Nodules in Congenital Nevi. Although melanomas may occur in the dermal component of congenital nevi, cellular proliferative nodules that occur in these nevi often do not behave in a clinically aggressive fashion (304). Such lesions may arise as one or more relatively small nodules that develop sometimes rapidly within a preexisting congenital nevus, particularly in infancy and early childhood. In a clinical pathologic study of a cohort of 26 cases, with a mean of 5-year follow-up on 16 patients, the lesions had an invariably benign clinical course. The features that are useful in differentiating cellular proliferative nodule from melanoma include: (a) lack of high-grade uniform cellular atypia; (b) lack of necrosis within the nodule; (c) rarity of mitoses; (d) evidence of maturation in the form of blending or transitional forms between the cells in the nodule and the adjacent nevus cells; (e) lack of pagetoid spread into the overlying epidermis; and (f) no destructive expansile growth (305). If some of these features are present in slight degree, the descriptive diagnosis of “melanocytic tumor of uncertain potential” may be appropriate (p. 930). Cases of cellular nodules in congenital nevi demonstrate chromosome loss with patterns that differ from the findings in frank melanomas (306), and most of them have mutations of NRAS rather than BRAF, considered to be characteristic of lesions that develop in the absence of sun exposure (285).
Leptomeningeal Melanocytosis. There is a diffuse infiltration of the leptomeninges with pigmented melanocytes. Also, the blood vessels entering the brain and spinal cord may be surrounded by melanocytes, and there may be areas of infiltration of the brain or spinal cord with melanocytes. Leptomeningeal melanoma can infiltrate the leptomeninx and form multiple nodules in the brain (307,308).
Principles of Management of Congenital Nevi. Excision of giant congenital nevi is suggested by some groups; however, heroic surgical procedures are often required, so that cosmetic improvement may be debatable. Because removal is not often complete and because of the risk of neurocutaneous melanosis, lifelong follow-up may be reasonable to consider even after efforts at surgical excision. In a registry-based study of 301 families, there was a high level of satisfaction with surgery in the patients who had small or intermediate facial lesions. This was significantly reduced with increasing lesion size. There was no evidence that surgery reduced the incidence of adverse clinical outcomes in childhood. The natural history of the majority of untreated congenital nevi was to lighten spontaneously, whereas some treatments may cause adverse effects (290). On the basis of considerations like these, many groups opt for follow-up over surgical removal of these lesions.
The excision of all non-giant congenital nevi, where feasible, is often considered (260,309). However, the risk of melanoma in any given lesion is small and not limited to childhood (310). In an individual within only a single small or intermediate congenital nevus, excision may be a simpler alternative to follow-up, or alternatively, a patient could be given guidelines for self-examination. The small “congenital pattern nevi” discussed earlier are probably best managed as for common acquired nevi, namely, by excision to rule out melanoma only for lesions that are changing or otherwise atypical.
Deep-Penetrating Nevus (Plexiform Spindle Cell Nevus)
This is a distinctive entity that has some features of combined nevus, blue nevus, and Spitz nevus (311,312). In the first report of 70 cases from a referral center, many cases had previously been misdiagnosed histologically as melanomas. Similar lesions have also been described as plexiform spindle cell nevi (54,55,313).
Clinical Summary. Most of the lesions occurred in the second and third decades (range 3 to 63 years). The head, neck, and shoulder were the most frequent sites of involvement, with no occurrences on the hands or feet. The lesions ranged from 2 to 9 mm, and were darkly pigmented papules and nodules, often diagnosed clinically as blue nevi or cellular blue nevi. In a mean follow-up of 7 years, none recurred or metastasized. Occasional recurrences, but no metastases, have since been described (314). On cross-section, the lesions extended at least halfway into the dermis, with a smooth, dome-shaped elevation of the epidermis. It is possible that lesions termed “nevi with focal atypical epithelioid component” or “clonal” or “combined” nevi may represent a superficial variant (314). These distinctions are of little or no importance; the key distinction is from melanoma.
Histopathology. At scanning magnification, the lesions are circumscribed and pyramidal in shape, with a broad base abutting the epidermis, and an apex extending into or toward the fat (Fig. 28-15). Nests of nevus cells at the dermal–epidermal junction are usually present. The dermal component is composed of loosely arranged nests or plexiform fascicles of large pigmented spindle and epithelioid cells interspersed with melanophages. There is a tendency to formation of narrow spindle cells at the periphery of the nests, reminiscent of sustentacular cell differentiation. In many cases there is an admixture of smaller, more conventional nevus cells. The lesional cell nests tend to surround skin appendages, and to infiltrate the collagen at the periphery of the lesion. The cells do not tend to “mature” with descent into the dermis. Some lesions have a patchy mild lymphocytic infiltrate.
Figure 28-15 A: Deep-penetrating nevus. At scanning magnification, the lesion is pyramidal, with its base applied to the epidermis, and its apex in the reticular dermis. B: The lesional cells in the dermis are arranged in nests and plexiform fascicles. They may be heavily pigmented and there may be scattered large, hyperchromatic, and pleomorphic nuclei, constituting “random” cytologic atypia. Mitoses are absent, or very rare. C: The lesions may span the reticular dermis and involve the subcutis. D: Scattered lesional cells may be enlarged, irregular, and hyperchromatic, constituting “random” cytologic atypia. There may be a suggestion of suscentacular cell differentiation at the periphery of some of the nests. Mitotic figures are absent in most lesions, or very rare.
At higher magnification, nuclear pleomorphism may be striking in some lesions, with variation in size and shape, hyperchromasia, and nuclear pseudo-inclusions. The atypia tends to be confined to randomly scattered cells rather than being “uniform,” or present in a majority of the cells. Nucleoli are usually inconspicuous but a few large eosinophilic nucleoli may be observed. Importantly, mitoses are absent or very rare, with no more than one or two in multiple sections of any given lesion. The cytoplasm is abundant, and contains finely divided brown melanin pigment. The lesional cells react positively for S-100 protein and HMB-45 antigen (313).
Some lesions in the general category of deep-penetrating nevi have histologic features that overlap with those of melanomas, including a tendency to sheet-like rather than nested fascicular differentiation, the presence of more than a rare mitosis, and increasing degrees and greater uniformity of atypia. Some of these lesions have recurred locally, metastasized to regional nodes or, in rare cases, perhaps systemically. Some of these lesions have recurred locally, metastasized to regional nodes, or, in rare cases, perhaps beyond. Such lesions have been placed into a category of “borderline melanocytic tumors” (315) or may be considered as “MELTUMP” (316).
Differential Diagnosis. Deep-penetrating nevi can be distinguished from nodular melanoma by architectural and cytologic features. Most bulky tumorigenic melanomas exhibit a more striking pattern of epidermal involvement with spread of atypical cells into the epidermis and, often, with ulceration. Melanomas are likely to be more broad than deep, while deep-penetrating nevi tend to be vertically oriented like Spitz nevi. Tumorigenic melanomas usually exhibit a more destructive pattern of infiltration, with displacement and compression of the stroma, and often with necrosis. Most also display marked nuclear atypia with frequent and often abnormal mitoses. A few nodular spindle cell melanomas have lower grade nuclear atypia, and in these cases, the presence of more than a few mitoses may be decisive. A study found rates of expression of the cell cycle proliferation marker PCNA to be somewhat higher in deep-penetrating nevi than in ordinary banal nevi, but considerably lower than in melanomas (317).
Benign lesions that may show some tendency to overlapping features with deep-penetrating nevi include common and cellular blue nevi, as well as spindle and epithelioid cell nevi, and clonal or combined nevi (314). Although deep-penetrating nevi can usually be distinguished from these benign or low-grade lesions, the distinction from melanoma is of greatest importance. Neural involvement is not an indicator of malignancy in these lesions.
Principles of Management. Although most deep-penetrating nevi will have a benign clinical course, sufficient numbers have not been studied in our opinion to insure this with certainty, and we recommend complete excision of such lesions (47). As with Spitz nevi, this approach also minimizes the possibility of local recurrence of a nevus variant with intrinsically unusual features in scar tissue, a situation at potential risk for overdiagnosis and overtreatment as outright melanoma.
Halo Nevus
A halo nevus, also known as Sutton nevus or nevus depigmentosa centrifugum, represents a pigmented nevus surrounded by a depigmented zone, or halo (Fig. 28-16A).
Figure 28-16 A: Halo nevus clinical. The halo develops around a preexisting unremarkable compound nevus. B: Halo nevus. A dense infiltrative lymphocytic response blurs the silhouette of the lesional nevus cells in the dermis at scanning magnification. C: Halo nevus. Small lymphocytes are diffusely placed among the dermal nevus cells, which may appear swollen and slightly atypical (“reactive” atypia). Severe or uniform atypia or mitotic activity should suggest the possibility of melanoma. D: Halo nevus. At the periphery of the nevus, the halo is a region where pigment and melanocytes are reduced or absent, and there may be a subtle lymphocytic infiltrate at the dermal–epidermal junction, as here.
Clinical Features. The nevus may be of almost any of the types described in the preceding sections, and a similar halo reaction may be seen rarely in relation to primary or metastatic melanoma. In the common type of halo nevus, which is characterized histologically by an inflammatory infiltrate and is therefore referred to as inflammatory halo nevus, the central nevus only rarely shows erythema or crusting; however, it undergoes involution in most instances, a process that may extend over a period of several years (318). The area of depigmentation shows no clinical signs of inflammation and, even though it may persist for many months and even years, it ultimately repigments in most cases. Halo nevi tend to progress through several clinical stages. The classic early lesion is a brown nevus with a surrounding usually symmetrical rim of vitiligo-like depigmentation. The central nevus may then lose its pigment and appear pink with a surrounding halo; the central papule may then disappear leading to a circular area of macular depigmentation, or the depigmented area may repigment, leaving no trace of its prior existence. In unusual lesions, darkening of the central nevus rather than lightening has been described (319). Most persons with halo nevi are children or young adults, and the back is the most common site. Not infrequently, halo nevi are multiple, occurring either simultaneously or successively.
Besides the more common inflammatory halo nevus with histologically apparent inflammation, there also are cases of noninflammatory halo nevi in which histologic examination shows no inflammatory infiltrate (320). In such instances, the nevus does not involute. In addition, there is the so-called halo nevus phenomenon, also referred to as halo nevus without halo. In these instances, the nevus shows histologic signs of inflammation analogous to a halo nevus but without presenting a halo clinically (321). Such nevi may or may not involute. An association with Turner syndrome has been described (322).
Halo dermatitis around a melanocytic nevus refers to a temporary inflammatory reaction surrounding a nevus (Meyerson eczematous nevus) (323). There is a papular compound nevus that becomes surrounded by an eczematous halo. Histologically, the epidermis adjacent to the nevus is spongiotic. An analogous phenomenon of “nevocentric” erythema multiforme has also been described where cytotoxic/interface alterations predominate (324). Similar changes may be seen around atypical or dysplastic nevi (325).
Histopathology. An inflammatory halo nevus in its early stage shows nests of nevus cells embedded in a dense inflammatory infiltrate, in the upper dermis, and at the epidermal–dermal junction (Fig. 28-16B–D). Later, scattered nevus cells tend to predominate over nests. Even when melanin is still present in the nevus cells, these cells often show evidence of damage to their nucleus and cytoplasm, and some frankly apoptotic nevus cells are commonly observed. Some cells, especially superficially, may have enlarged ovoid nucleoli, changes that may be regarded as a form of “reactive atypia.” High-grade nuclear atypia is not observed. Importantly, the lesional cells tend to show evidence of “maturation,” becoming smaller with descent from superficial to deep within the lesion. Nevus cell mitoses are rare, and if present, should prompt consideration of the possibility of melanoma. In this regard, it may be difficult to differentiate nevus cell mitoses from those attributable to the reactive mononuclear cells, a problem we have found to be lessened by use of a combined (dual) Mib-1 (Ki-67) and Melan-A/MART-1 stain. Most of the cells in the dense inflammatory infiltrate are lymphocytes. However, some of them are macrophages, in which varying amounts of melanin are contained. As the infiltrate invades the nevus cell nests, it often is difficult to distinguish between the lymphoid cells of the infiltrate and the type B nevus cells in the mid-dermis, because they, too, may have the appearance of lymphoid cells. Immunohistochemical studies using S100 or a more specific marker such as Melan-A may be very helpful in identifying the nevus cells in the infiltrate (326). The infiltrate tends to extend upward into the lower portion of the epidermis. In most instances, the infiltrate is characterized by dense cellular packing without vasodilatation or intercellular edema and by sharp demarcation along its lower border.
At a later stage, only a few and finally no distinct nevus cells can be identified. Gradually, after all nevus cells have disappeared, the inflammatory infiltrate subsides. Despite the clinical evidence of regression and the inflammatory infiltrate that consists predominantly of T cells (327), fibrosis is not a prominent feature (328). The inflammation is occasionally granulomatous (329).
In both inflammatory and noninflammatory halo nevi, the epidermis of the halo at first shows a reduction in the amount of melanin on staining with silver and fewer dopa-positive melanocytes than are seen in the normal epidermis. Ultimately, there is complete absence of melanin and also a negative DOPA reaction. Especially in early lesions, lymphocytes may be seen rosetting around damaged melanocytes in the halo.
In Meyerson eczematous nevus, a junctional or compound nevus is associated with a symmetrical area of epidermal acanthosis, spongiosis, parakeratosis, and a dermal infiltrate of lymphocytes, histiocytes, and eosinophils. Immunohistochemical analysis reveals that the lymphocytes are CD3(+) with a predominance of CD4(+) cells in comparison to CD8 (330).
Histogenesis. Immunohistochemical staining for S100 protein helps in the identification of nevus cells within the inflammatory infiltrate, because their number may be small and it may be difficult to differentiate them from the lymphocytes of the infiltrate (326). However, Langerhans cells and some histiocytes also react with S100 protein and therefore, a more specific marker such as Melan-A is preferable. Electron microscopic study reveals that nevus cells and melanocytes within reach of the infiltrate are damaged and ultimately disappear. In the nevus, many nevus cells appear vacuolated and contain only few melanosomes, but large aggregates of melanosomes are seen within macrophages (331,332).
In the depigmenting halo, the melanocytes show various kinds of degeneration, such as vacuolization and coagulation of the cytoplasm and autophagocytosis of melanosomes (333). It has been suggested that an initial noncellular stage of inhibition of both the melanocytes and the nevus cells may be responsible for the development of the depigmented halo, preceding the appearance of a dermal lympho-macrophagic infiltrate that ultimately leads to the destruction of the nevus (331). Both in halo nevi and in vitiligo, the depigmentation takes place through disappearance of the melanocytes. However, despite occasional patients who present with both lesions, halo nevus is likely not a form of vitiligo (334). The abundance of potential antigen-presenting cells and lymphocytes (including CD8 positive T cells) in the regressing nevus at the site of depigmentation suggests that these cells participate in the destruction of nevus cells and melanocytes in the halo phenomenon (335). In an interesting study of the lymphoid elements infiltrating halo nevi, oligoclonal expansion of T cells was observed in all patients, and in one patient, T cells using the same TCR beta chain were observed in distinct halo nevi, demonstrating a local expansion of common clones that are most likely activated by shared antigens within the nevi (336).
Differential Diagnosis. It can be difficult to differentiate early lesions of inflammatory halo nevus from a melanoma; both types of lesions may have a dense cellular infiltrate in the dermis, and, in halo nevi, the nevus cell nests, as a result of having been invaded by the cellular infiltrate, may appear atypical, which we consider to be “reactive.” The danger of misinterpretation is greatest in halo nevi without a halo, the so-called halo nevus phenomenon. However, the inflammatory infiltrate in halo nevi is more pronounced than in melanoma and extends diffusely through the lesion, rather than being concentrated at the periphery as in most examples of tumorigenic melanoma. The diagnosis of melanoma rather than halo nevus is likely in a complex lesion that has an adjacent in situ or microinvasive component. Whether or not such an adjacent component is present, attributes of the nodule itself that should prompt consideration of melanoma include larger size, asymmetry, increased cellularity, lack of lesional cell maturation, uniform high-grade nuclear atypia, and mitotic activity.
If no identifiable nevus cells are present, the diagnosis of halo nevus may be suggested by the presence of melanophages in the dense cellular infiltrate and by the absence of melanin in the epidermis on staining with silver. However, one should also consider the possibility of a regressed squamous lesion or melanoma.
Principles of Management. Halo nevi and related lesions generally do not require active therapy. Lesions are often excised for cosmetic reasons, and to rule out melanoma. A halo nevus is not a risk factor for future development of melanoma.
Recurrent Nevus (Pseudomelanoma)
Recurrence of a nevus may show clinical hyperpigmentation, which on biopsy may show histologic changes suggestive of melanoma (337–341).
Clinical Features. Recurrence may follow incomplete removal of a nevus, particularly by a shave biopsy or electrodessication, or the nevus may apparently have been completely excised. A similar phenomenon has been described in relation to Spitz tumors (342). The term “persistent nevus” is sometimes used because it is considered that the clinical “recurrence” must have arisen from nevus cells that were not removed by the prior procedure. However, we have also considered the possibility that the “recurrent” pigmentation might be the result of a reactive hyperplasia and nevoid transformation of melanocytes in the epidermis above the healing biopsy site as a consequence of melanocyte growth factors known to be elaborated by scar tissue (e.g., c-kit ligand or fibroblast growth factors). In any event, at a clinical level, recurrence of apparently completely excised nevi is common. In a prospective follow-up study, 28% of all nevi and 41% of hairy nevi were reported to have recurred within 12 months after shave excision (343). The pigmentation in recurrent nevi is confined to the region of the scar and typically presents within a few weeks of the surgical procedure (337). After this rapid appearance, the pigment is stable. In contrast, recurrent melanoma does not respect the border of the scar, and extends over time into the adjacent skin. Paradoxically, recurrent melanoma occurs more slowly, over months or years, but progresses inexorably.
Histopathology. Although most recurrent nevi are not cytologically atypical, in a few instances they contain atypical melanocytes, both singly and in nests, arranged mainly along the epidermal–dermal junction, but occasionally also extending into the upper dermis and also into the epidermis in a pagetoid pattern (163) (Fig. 28-17). The junctional nests are often composed of pigmented epithelioid melanocytes forming irregular nests, possibly the result of their growth within an atrophic epidermal layer that interfaces with scar tissue. The rete ridge pattern may be preserved or effaced (344). Deep remnants of the nevus may be seen in the reticular dermis beneath the scar (341). A lymphocytic infiltrate with melanophages may be seen in the upper dermis. The fibrosis of the scar may mimic and be mistaken for “regressive fibrosis” in a melanoma, especially in a superficial shave biopsy where the bases of the scar cannot be visualized. Nevus cells in the dermis tend to show evidence of maturation, and the Ki-67 proliferation rate is low (345
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree