Figure 24-1 Acute cutaneous leishmaniasis. A: Ulceration with crust on the ear of a returning traveler from Costa Rica. (Photo courtesy of Dr. Misha Rosenbach, University of Pennsylvania, Philadelphia, PA.)
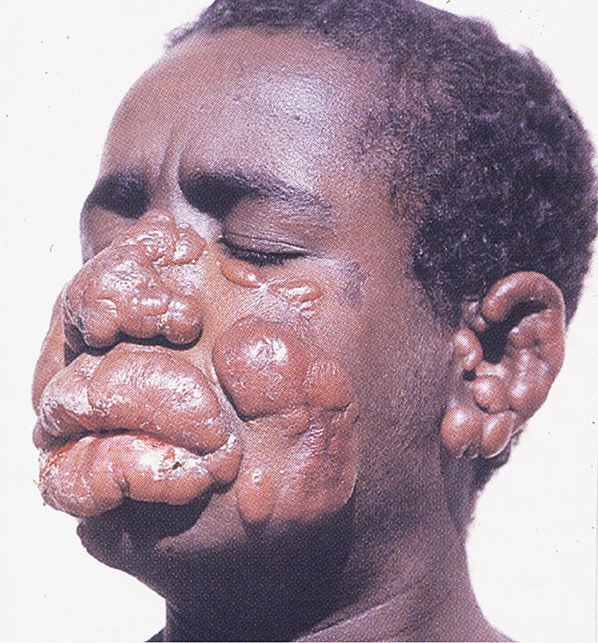
Figure 24-2 Diffuse acute cutaneous leishmaniasis. Ethiopian patient with pseudolepromatous appearance. (From Schaller KF, ed. Colour atlas of tropical dermatology and venerology. Berlin, Germany: Springer, 1994:113, with permission.)
ML, also known as espundia, is a disease almost exclusively of South America and is rare in the United States (29,30). It constitutes approximately 5% of cases of New World leishmaniasis. It is primarily caused by L. braziliensis and L. panamensis (31). Patients present with ML several months to years after developing CL. However, cases of CL presenting synchronously with ML have been reported (32,33). ML presents with chronic nasal congestion, nasal bleeding, ulceration, and septal granulomas. In addition to the nasal mucosa, it has been described to involve the lips, palate, mouth, pharynx, larynx, and middle ear (34–37). It is an aggressive form of leishmaniasis, often leading to destruction of the nasopharynx tissue.
VL, also known as kala azar, causes over 50,000 deaths a year according to the WHO. It is characterized by undulating fevers, weight loss, hepatosplenomegaly, lymphadenopathy, and cytopenias (38). Except in HIV-positive patients, it rarely has cutaneous findings outside of the skin darkening and an erythematous malar rash seen on the Indian subcontinent. Patients with HIV may display a variety of cutaneous findings, including a spindle cell pseudotumor, colonization of a Kaposi sarcoma tumor, and coinfection at the site of herpes zoster infection (39–42). A bone marrow smear is the reference method for diagnosing VL, the condition can be confirmed through additional molecular tests. Serology is sometimes used in regions where a bone marrow smear is not available. Patients with resolved kala azar may develop cutaneous lesions in a form of leishmaniasis termed post–kala azar dermal leishmaniasis (PKDL). PKDL is primarily associated with L. donovani. These patients present with hypopigmented macules which progress to flesh-colored or erythematous papules and nodules. This form may be confused with lepromatous leprosy. PKDL can be differentiated from the diffuse cutaneous form of CL by the history of previously treated or resolved VL in PKDL patients. PKDL has been a manifestation of the immune reconstitution inflammatory syndrome (IRIS) in HIV patients upon the initiation of highly active antiretroviral therapy (HAART) and in transplant patients (43,44).
Cases of leishmaniasis are increasing. This is thought to be a result of global climate change, increased range of the phlebotomine sandflies, increased international travel, armed conflicts, the worldwide HIV epidemic, and increased iatrogenic immunosuppression (45). Transmission of leishmaniasis has been reported through blood transfusion (46). HIV is clearly a predisposition to more severe and atypical cases of Leishmania. Several cases of Leishmania presenting in patients undergoing treatment with TNF inhibitors have been reported (47–49).
A variety of ancillary testing is available for leishmaniasis. Intradermal testing for leishmanin (Montenegro test) is available in some parts of the world. Leishmania can be cultured on Novy-MacNeal-Nicolle media or by animal inoculation. Organisms can be identified through isoenzyme analysis or other molecular methods. Identification of the causative species has become easier with the introduction of PCR testing (50). PCR is especially useful in cases of CL and ML where the overall number of organisms may be low. This testing can be done on fresh tissue as well as formalin-fixed, paraffin-embedded tissue. One of the most sensitive PCR methods targets the minicircle kinetoplast DNA (kDNA) (51,52).
Histopathology. All of the different species of Leishmania present with an identical morphology on microscopic exam. Organisms are 2 to 4 μm in size and round to oval in shape. They are notable for their nucleus and kinetoplast. The kinetoplast is a rod-shaped organelle that contains extranuclear DNA in maxi- and minicircles. Organisms are generally found within tissue macrophages but can be found extracellularly, especially in cases with high organism numbers. In the histiocyte, the organisms often stud the inner membrane in a “marquee” pattern. Amastigotes can be highlighted on Giemsa staining where the cytoplasm stains light blue and the nucleus and kinetoplast stain pink-red or violet. They do not stain with Grocott methenamine silver stain or periodic acid–Schiff stain.
In CL, there is a dense mixed dermal inflammatory infiltrate predominantly of histiocytes, lymphocytes, and plasma cells (Fig. 24-3). Giant cells and eosinophils may also be seen, and neutrophils are noted once ulceration has occurred. Parasitized histiocytes are appreciable within the inflammation (Fig. 24-4A, B) (53–55). In early lesions, there may be overlying acanthosis or atrophy. In later lesions, ulceration and pseudoepitheliomatous hyperplasia may develop, and organisms may be difficult to find due to decreased numbers. There is an increase in the number of tuberculoid granulomas, but perineural involvement and caseating necrosis are rarely seen (56,57). Tuberculoid granulomas are prominent in recidivous or lupoid disease.
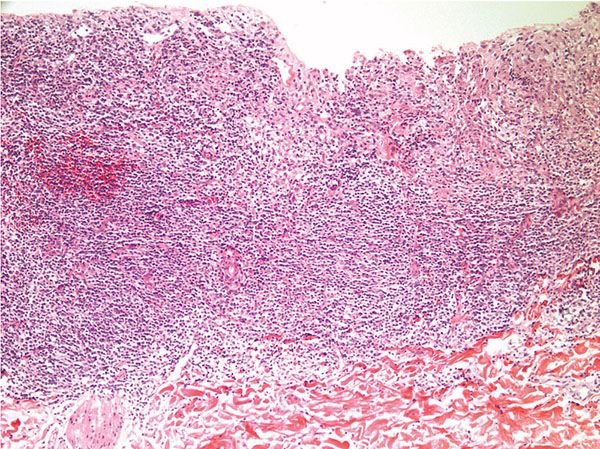
Figure 24-3 Leishmaniasis. Low-power view of ulcerated lesion of CL with dense mixed inflammatory infiltrate.
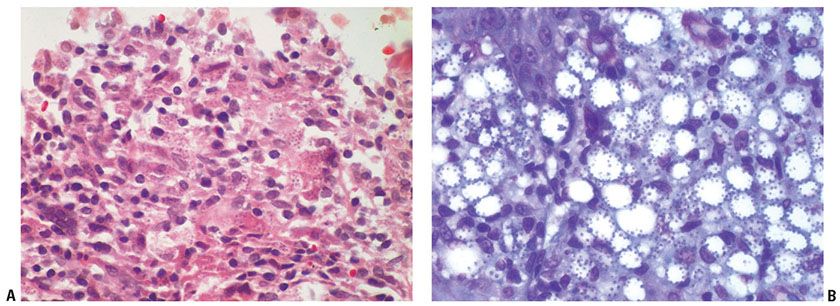
Figure 24-4 Leishmaniasis. A: High-power view of ulcerated lesion of CL with mixed inflammatory infiltrate and parasitized histiocytes. B: Giemsa stain of CL with “marquee” sign of organisms lining the internal cell membrane of the histiocytes.
The histopathologic features of ML are similar to the early and late stages of CL. Sangueza et al. (58) subdivide the histologic features into edematous, granulomatous, and necrotizing granulomatous stages. Suppuration and necrosis may be seen more frequently than in CL (58). Fibrosis can be seen in the dermis in long-standing lesions.
Cutaneous lesions in VL are rare. These lesions are characterized by a perivascular lymphohistiocytic infiltrate with parasitized histiocytes (59). Parasites have been noted in the eccrine glands, colonizing a tumor of Kaposi sarcoma and forming a spindle cell pseudotumor in other cases (39–41). There are few reports on the histopathology of PKDL. In a large case series looking at the dermal nodules in PKDL, there was an atrophic epidermis, prominent follicular plugging, a dense lymphohistiocytic inflammatory infiltrate, and collagen alteration (60). The authors of this case series highlight the importance of a negative Fite stain to rule out lepromatous leprosy. Similar findings of a dense lymphohistocytic infiltrate in the dermal nodules and a superficial perivascular infiltrate in the macular hypopigmented lesions have been reported by other groups (61–64).
Pathogenesis. As noted above, leishmaniasis is dependent not only on the type of Leishmania spp. but also on the host. Amastigotes disrupt macrophage activation, which leads to their persistence (65). Studies in mouse models of Leishmania and in humans have shown the importance of HLA and components of the cytokine and chemokine pathway in infection (66,67). Classically, resistance to disease progression has been associated with a Th1 response and susceptibility with a Th2 response. This is likely a much more complex issue with important contributions from Th17 and regulatory T cells (68).
Differential Diagnosis. The main differential is with the other infections that parasitize macrophages: histoplasmosis, Klebsiella granulomatosis (granuloma inguinale), Klebsiella rhinoscleromatosis (rhinoscleroma), and penicilliosis. Special stains and the identification of the kinetoplast can be helpful in identifying Leishmania. Leishmania lacks the capsule seen in Histoplasma capsulatum. As lesions become more granulomatous, the differential diagnosis shifts to including other granulomatous conditions such as tuberculosis, sarcoidosis, and granulomatous rosacea. PCR may be helpful in identifying the correct diagnosis, especially in these cases.
Principles of Management. Treatment depends on the host, the parasite, and the form of leishmaniasis infection. Expert advice in planning the best treatment regimen is advised. Systemic therapies with good reports of effect include miltefosine, liposomal amphotericin B, conventional amphotericin B, and pentavalent antimony (sodium stibogluconate). Secondary agents include paromomycin, azole family antifungals, and pentamidine (69). Treatments for localized disease include cryotherapy, heat therapy, topical paromomycin, and intralesional sodium stibogluconate (70). ML and VL are always treated. CL is treated to hasten resolution, reduce scarring, decrease chance of recurrence, and attempt to prevent mucosal disease; in general, patients in the United States with CL will receive therapy (69). Patients should be made aware of the chance of treatment failure and, despite satisfactory clinical response, the chance for relapse and recurrence, including delayed relapse many months or even years after the initial infection.
AMEBIASIS
Clinical Summary. Human cutaneous amebiasis can be segregated into two main categories: infections caused by the parasitic Entamoeba spp. and infections caused by the free-living amebae (Acanthamoeba, Naegleria fowleri, and Balamuthia mandrillaris) (71). Amebae are single cell organisms that exist in either the environmentally stable cyst form or the pathogenic trophozoite form. As opposed to free-living amebae that are considered facultative parasites of humans, Entamoeba histolytica is endemic in parts of Africa, Asia, and South America. It is reported to infect approximately 10% of the worldwide population although this number may be lower as molecular diagnostic techniques identify new subspecies of Entamoeba.
E. histolytica primarily infects the gastrointestinal tract. Cutaneous lesions typically result from either direct extension to the perianal and genital skin or through fistulas to the skin from underlying GI or hepatic abscesses. Primary cutaneous lesions have been reported but are rare (72). Typical lesions are painful and malodorous ulcers with a gray-white necrotic base (73). Perianal and genital lesions are often verrucous and may be mistaken for genital warts or squamous cell carcinomas (74). Venereal transmission has been reported. More advanced disease has been associated with HIV infection.
Free-living amebae are most commonly associated with devastating CNS infections. Acanthamoeba and Balamuthia cause a granulomatous encephalitis whereas Naegleria causes a meningoencephalitis. Acanthamoeba is a causative organism of keratitis, especially in contact lens wearers (71). Cutaneous lesions have been described as pustules, chronic ulcers, and nodules. Chronic plaques and ulcers of the central facial region have been especially noted in Balamuthia mandrillaris infections in South America (75). Sporotrichoid spread has also been reported. HIV-positive and immunosuppressed patients have increased susceptibility (76,77). The skin involvement can be as a result of direct inoculation or result from underlying visceral disease.
Histopathology. Infections with E. histolytica demonstrate ulceration with necrosis and a mixed inflammatory infiltrate. Extravasated erythrocytes are often seen (78). E. histolytica is approximately 20 to 50 μm in size, round, and basophilic. There is an eccentrically placed nucleus and central karyosome (79). Erythrocytes are commonly noted within the cytoplasm. PAS stain can be useful in highlighting the organism. As noted above, there may be pseudoepitheliomatous hyperplasia at the edge of the lesion or in place of the ulceration leading to confusion with verrucous carcinoma.
Free-living amebae tend to form a granulomatous infiltrate seated in the deep dermis and subcutaneous tissue. Granulomas may be ill-defined to tuberculoid in appearance. There is often a surrounding lymphoplasmacytic infiltrate. Organisms can be identified within the granulomas. Acanthamoeba, Balamuthia mandrillaris, and Naegleria fowleri are described as 8 to 40 μm, 50 to 60 μm, and 10 to 25 μm in size in the trophozoite stage, respectively (71). Acanthamoeba has a centrally placed nucleus and nucleoli without erythrocytes or debris within the cytoplasm (Fig. 24-5) (80). As opposed to E. histolytica, vasculitis is often appreciated (80). These organisms can be identified in the vessel walls in CNS disease suggesting that the organisms may directly contribute to the vasculitis. It is important to note that, in patients with AIDS or on immunosuppression, granulomas may not be present. Less commonly, these infections can present with ulceration and neutrophilic abscesses. Suppurative panniculitis has been described associated with Acanthamoeba (80–82). Ulceration with necrosis and surrounding pseudoepitheliomatous hyperplasia, similar to the ulcers seen in E. histolytica infection, can be seen in the cutaneous ulcers of Balamuthia mandrillaris. Immunofluorescent and immunohistochemical stains are available for these organisms but are generally only available at specialized institutes such as the Center for Disease Control and Prevention (Atlanta, GA, USA).

Figure 24-5 Acanthamoebiasis. The trophozoite is identified by the central nucleus and prominent nucleoli within the fibrosis and inflammatory infiltrate.
Differential Diagnosis. Because of the pseudoepitheliomatous hyperplasia seen in E. histolytica, it may be mistaken for verrucous carcinoma.
The granulomatous inflammation associated with free-living ameba is similar to that seen in other infectious processes, especially in leishmaniasis and tuberculosis, as well as other granulomatous conditions such as granulomatosis with polyangiitis (formerly Wegener’s), sarcoidosis, and lymphomas. Ameba may be mistaken for macrophages or fungal organisms if the distinctive eccentric nucleus and nucleoli are not identified.
Principles of Management. Metronidazole is the treatment of choice for E. histolytica infections. Other options include diloxanide, tinidazole, and pentamidine. Numerous therapies have been tried for free-living amebae but none have proved reliably effective. This may change with the introduction of miltefosine which has been effective in the treatment of cases of Acanthamoeba and Balamuthia (83,84). The CDC now carries a stock supply, since it is not yet FDA approved, for these infections. In addition, the CDC recommends a combination of other treatments in these cases such as amphotericin B, azithromycin, and rifampicin (85).
SCABIES
Clinical Summary. Acarid mites produce several skin manifestations in humans, the most common one represented by scabies, which is caused by the eight-legged itch mite Sarcoptes scabiei var. hominis, also referred to as S. sarcoptes scabiei. Animal pathogenic mites can also affect the skin, although most commonly through bite reactions and not human infestation (1).
Burrows, which represent a tunnel through which a female mite travels to lay her eggs, are the pathognomonic lesions of scabies and are found mostly in the florid, papulovesicular type infestation (Fig. 24-6A). They occur mainly on the palmar and lateral aspects of the fingers and toes, the interdigital spaces, the flexor surfaces of the wrists, the nipples of women, the genitals of men, and, to a lesser extent, on the buttocks and axillae. Characteristically, the head is spared, except in infants, elderly, and immunocompromised (86). The burrows appear as fine, tortuous, grayish threads a few millimeters long. A vesicle may be visible near the blind end of the burrow. The mite, eggs, or feces may be situated at the end of the burrow and are sometimes visible by dermatoscopy (87). Mites and their biproducts have also been easily identified using reflectance confocal microscopy in crusted scabies, and a relatively homogenous distribution has been noted in this condition over all body surfaces (88). Although pathognomonic, the burrow is not the most common lesion seen in scabies. Small, erythematous, often excoriated papules are more frequent (86).

Figure 24-6 Scabies. A: Numerous pustules and papules are noted on the back of the foot and the toes of this infected child. B: Adult female mite found in skin scrapings. C: The female mite is located in the upper portion of the epidermis underneath the stratum corneum.
In some patients, itching nodules persist for several months after successful treatment, and therefore are named nodular scabies or persistent nodules. They are found most commonly on the scrotum and are believed to result from a prolonged response to persistent scabies antigens (89). In children, sometimes these nodules may grow so large they can be mistaken for infectious or malignant lesions.
In a third, rare variant called crusted scabies (formerly known as Norwegian scabies), innumerable mites are present. Patients with this variant show widespread erythema, hyperkeratosis, crusting, nail thickening, and subungual hyperkeratosis but no obvious burrows.
Histopathology. A definitive diagnosis of scabies can be made only by demonstration of the mite or its products (eggs or feces). A very superficial epidermal biopsy or scraping of an early papule or, preferably, of an entire burrow may be carried out with a no. 15 scalpel blade (90). The specimen is placed on a glass slide and a drop of immersion oil is placed on top of it (Fig. 24-6B).
Histologic examination of a biopsy containing a burrow reveals that the burrow in almost its entire length is located within the stratum corneum (91). Only the extreme, blind end of the burrow, where the female mite is situated, extends into the stratum malpighii (92). The mite has a rounded body and measures about 350 to 450 μm in length and 250 to 350 μm in width (Fig. 24-6C) (1). The presence of scabies can be made presumptively by the presence of pink pigtail-like structures, which are likely remnants of egg shells, within the intracorneal burrow (Fig. 24-7) (93). Polaroscopy may also be a helpful tool, given that attached and detached spines, as well as feces (scybala), are polarizable (94).
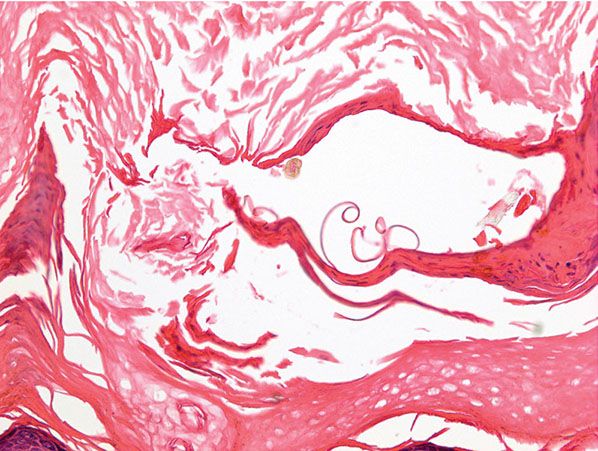
Figure 24-7 Scabies. Presence of scabies can be made presumptively by the presence of pink pigtail-like structures, which are likely remnants of egg shells.
In the papulovesicular form of scabies, spongiosis is present in the stratum malpighii near the mite to such an extent that formation of a vesicle is often the result. Even if no mite is found in the sections, the presence of eggs containing larvae, egg shells, or scybala within the stratum corneum is indicative of scabies (91,95). The dermal infiltrate in sections containing mites shows varying numbers of eosinophils.
In nodular or persistent nodular scabies, there is a dense, chronic inflammatory, often pseudolymphomatous infiltrate in which many eosinophils may be present. Vasculitis is considered by some as being frequent (95) but by others as representing a rather uncommon event (89). These different findings may be related to the duration of the scabietic nodules and the timing of the biopsy (89). The nodules are rich in indeterminate cells, sometimes misleading to the diagnosis of Langerhans cell histiocytosis based on light microscopy and immunophenotyping alone (96). Atypical mononuclear, CD30+ cells may be found (97), and in some instances, the nodules show, as in persistent arthropod bites or stings (see later discussion), a histologic picture resembling that of lymphoma (98). Viable mites are hardly ever found in the nodules. However, mite parts are seen in up to 22% of cases (89).
Other inflammatory patterns have been described in association with scabies infestation, including an interstitial histiocytic pattern mimicking granuloma annulare (99).
In crusted scabies, the thickened horny layer is riddled with innumerable mites, so that nearly every section shows several parasites (Fig. 24-8) (95).
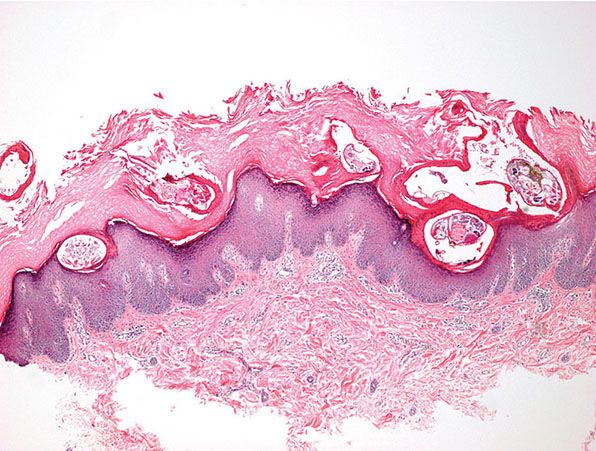
Figure 24-8 Crusted Scabies. Thickened stratum corneum is riddled with numerous mites, so that nearly every section shows several parasites.
Pathogenesis. Earlier scanning electron microscope studies revealed the keratinocytes around the burrow to be compacted, indicating that the mite physically forces its way in between the keratinocytes rather than chewing a passage (92). More recent studies, however, also using transmission electron microscopy, have found that the secretion of cytolytic substances by the mite as a contributing factor in advancing the parasite body through the skin in addition to mere compression (100). The cellular damage was greatest around the body, especially the mite capitulum.
Both cell-mediated and humoral immune responses are activated in scabies. The acute eczematoid reaction in the epidermis is indicative of cell-mediated hypersensitivity. A role for humoral hypersensitivity is suggested by the presence of immunoglobulin M and the third component of complement in vessel walls (89,101). Crusted scabies is observed generally in patients who are severely compromised in their immune responses, including those with leukemia, lymphoma, and the acquired immunodeficiency syndrome (102). Crusted scabies manifesting as an exuberant IRIS associated with HIV has also been reported (103).
Principles of Management. The optimal treatment depends on the type of disease, the patient’s age, and comorbidities. Topical medications, including permethrin 5% and malathion 0.5%, have proven excellent clinical success. In addition, oral ivermectin 200 μg/kg is a very effective treatment, particularly in patients with severe disease or crusted scabies. Combination therapy, and repeated treatments, are sometimes required in cases of extensive infestation or for immunocompromised hosts.
HOOKWORM-RELATED CUTANEOUS LARVA MIGRANS
Clinical Summary. Cutaneous larva migrans is caused by skin-penetrating larvae of nematodes, most commonly of the cat and dog hookworm Ancylostoma braziliense, A. caninum, A. tubaeforme, Uncinaria stenocephala, and Bunostomum phlebotomum (1,104–107). Other forms of cutaneous larva migrans are caused by human- and animal-type Strongyloides species and are then known as larva currens (see later discussion). Gnathostomum spinigerum does not belong to the hookworm family but to the group of spiruriids and can also evoke cutaneous larva migrans-like eruption (1).
Hookworm-related cutaneous larva migrans is the most common variant. The infestation occurs through contact with soil contaminated with the larvae. The exposed parts of the body, such as the feet, are most commonly involved, but any body part in direct contact with sand or soil may be affected. Creeping eruption represents the clinical manifestation of cutaneous larva migrans and is manifested by an irregularly linear, thin, raised, serpiginous burrow that is 2 to 3 mm wide (Fig. 24-9A). The larva and track may clinically be better visualized with the assistance of dermoscopy or reflectance confocal microscopy (108). The larva moves a few millimeters per day. The eruption is self-limited because humans are accidental hosts, leaving the hookworm incapable of sexually maturing.
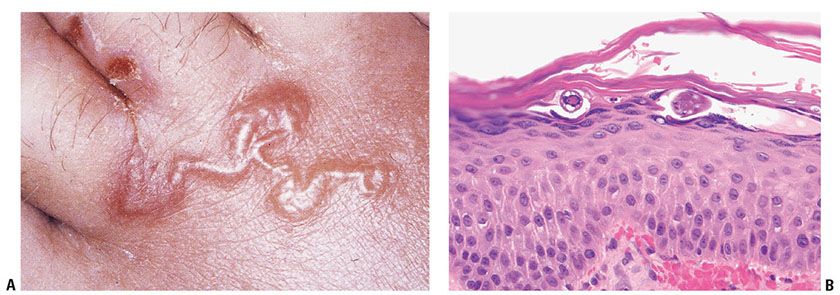
Figure 24-9 Cutaneous larva migrans. A: This curvilinear superficial burrow is the prototypical clinical manifestation. B: The organism is found underneath the stratum corneum in the upper portion of the stratum malpighii. (Specimen courtesy of Mandi Sachdeva, MD, and Rajiv Patel, MD, Cleveland Clinic Foundation, Cleveland, Ohio.)
Histopathology. The visible track does not correlate with the exact location of the larva and represents an inflammatory response composed of lymphocytes admixed with many eosinophils in the epidermis and upper dermis (1,105,106). The parasite is found 1 to 2 cm ahead of the visible track within a burrow located in the upper layers of the epidermis (Fig. 24-9B) (109). The lesion, aside from the larva—which is often not observed in the biopsies, shows spongiosis and intraepidermal vesicles in which necrotic keratinocytes can be seen. Numerous eosinophils in the superficial and deep reticular dermis are also commonly observed. A specific subtype of hookworm-related cutaneous larva migrans, which usually confined to the buttocks, is known as hookworm folliculitis (110). It is important to recognize this variant because the inflammation is typically perifollicular, the organisms are often deep within the follicles, and it is typically more difficult to treat (111).
Pathogenesis. The tissue penetration and the movements of the hookworm larvae within the epidermis are at least in part due to active production and secretion of proteases by the parasite (112). However, with the exception of hookworm folliculitis, these enzymatic activities are typically not adequate to allow penetration into the dermis, and hence the nematode is located in the upper portion of the epidermis (113).
Principles of Management. Cutaneous larva migrans is often self-limited, but severe pruritus and risk of superficial infection may be reason for treatment. Oral thiabendazole or ivermectin may be used.
STRONGYLOIDIASIS
Clinical Summary. Strongyloides stercoralis is a small intestinal nematode that is mainly endemic in the tropics and subtropics but is also found in the southeastern United States (especially eastern Kentucky, rural Tennessee, southern Virginia, and western North Carolina and South Carolina) (114). It is a geohelminth contracted by humans through cutaneous penetration of filariform larvae. A unique cycle includes traveling of the larvae via the venous system to the lungs, the patient coughing up and subsequently swallowing the larvae, and the parasite finding its final habitat in the small intestine (1). Three forms of strongyloidiasis can be delineated clinically: a not well characterized acute form, a chronic form, and a disseminated form (114). The chronic type presents with gastrointestinal and pulmonary symptoms and the pathognomonic larva currens. The latter represents a linear or serpiginous urticarial tract moving at a rate of 5 to 15 cm/hour caused by rapidly migrating larvae and is a subtype of cutaneous larva migrans (see previous discussion). It is believed to derive from autoinoculation with intestinal larva in the perineal area. The disseminated variant of strongyloidiasis occurs in the setting of severe immunosuppression and is commonly fatal. Immunosuppressive therapy for autoimmune disease, iatrogenic immunosuppression for transplant patients, human T-cell lymphotropic virus (HTLV), and AIDS may predispose to disseminated infection (115). Patients with advanced hyperinfection present with a purpuric skin eruption, often located periumbilically (116,117). Other cutaneous manifestations of strongyloidiasis include chronic urticaria, prurigo nodularis, and lichen simplex chronicus (116–118).
Histopathology. The serpiginous urticarial lesions believed to represent the migrating path of larvae are consistently negative for organisms (116). In contrast to hookworm-associated cutaneous larva migrans, the migratory tracts of larva currens are not located in the epidermis but in the dermis (1). It is only in the disseminated variant of strongyloidiasis that organisms can be found. They occur in the form of filariform larvae, measuring from 9 to 15 μm in diameter, which can be seen at all levels of the dermis. These larvae are commonly associated with vascular damage producing purpura and petechiae, which are likely due to vascular damage by the filariform larvae migrating through the vessel wall (Fig. 24-10A–C) (116,117,119,120). These changes often do not provoke a significant dermal inflammatory response, probably because these patients are severely immunocompromised (116,117,121) (Fig. 24-10A). Changes of leukocytoclastic vasculitis are also not noted (119–121). Eosinophil-rich granulomata around Strongyloides larvae are, however, occasionally described (114,120).
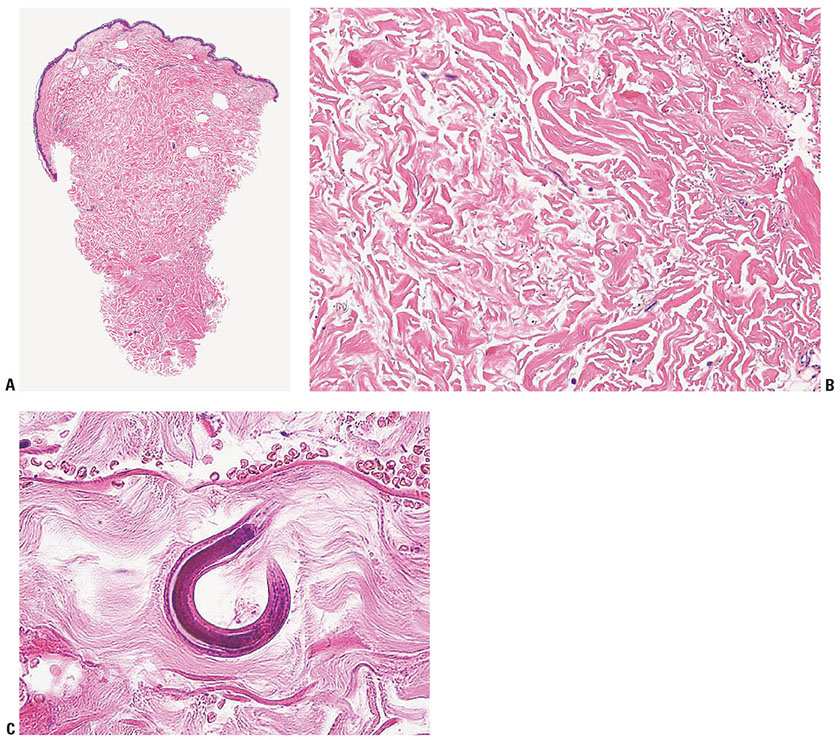
Figure 24-10 Strongyloidiasis. A: On scanning magnification, the punch biopsy from the periumbilical purpuric eruption shows no obvious histopathologic changes. The patient died from disseminated strongyloidiasis. B: On medium-power magnification, extravasated erythrocytes are noted (upper right corner) and basophilic linear and round structures become apparent. C: On rare occasions an entire nematode cut at the right angle is seen percolating between the collagen bundles. Note extravasated erythrocytes at the top of the micrograph. (A, B: Specimen courtesy of Arlene S. Rosenberg, MD, MetroHealth Medical Center, Cleveland, Ohio. C: Courtesy of Catherine M. Stefanato, MD, and Eduardo Calonje, MD, St. John’s Institute of Dermatology, London, UK.)
Principles of Management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








