Figure 18-1 Comedone, open. A dilated follicular infundibulum with an attenuated wall is plugged with keratin and sebum. The follicular orifice is widened.
Both open and closed comedones are associated with mild mononuclear inflammation around vessels in the adjacent papillary dermis. Attenuation of the follicular wall may be so extreme as to lead to rupture (3). Release of follicular contents into the dermis generates an inflammatory response initially mediated by neutrophils and later by histiocytes and foreign-body giant cells. When rupture occurs superficially, it tends to lead to the development of a clinical pustule (Fig. 18-2), but when it occurs in the deeper dermis, an inflammatory nodule forms (Fig. 18-3). If the follicular damage and inflammatory response are severe, large abscesses and scarring can result. This typically occurs in severe forms of acne (cystic acne and acne fulminans).
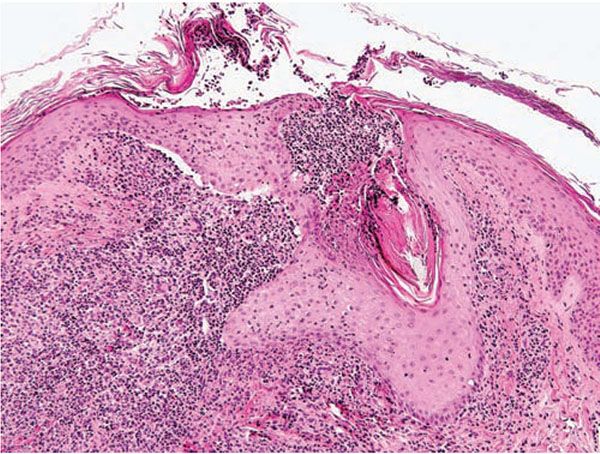
Figure 18-2 Acne vulgaris, pustule. Superficial follicular rupture incites suppurative and granulomatous inflammation, with neutrophils predominating in early lesions.
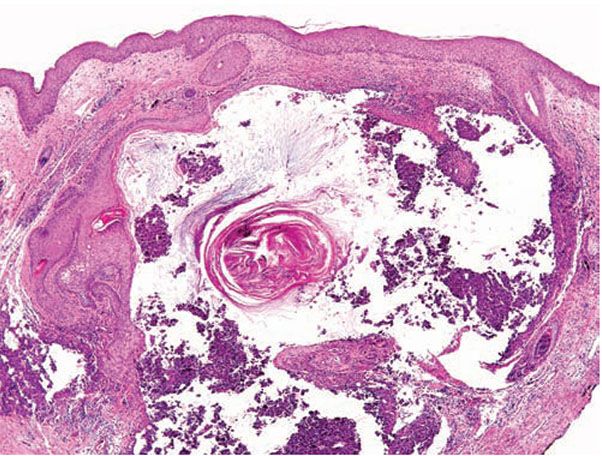
Figure 18-3 Acne vulgaris, nodule. Cystic follicular dilation with deep rupture leads to neutrophilic and granulomatous inflammation throughout the dermis.
Pathogenesis. Several reviews highlight important advances that have affected our understanding of acne (4,5). Acne vulgaris is a multifactorial condition, initially requiring sex hormone release during puberty and activation of the sebaceous glands. The major factors contributing to acne development are follicular hyperkeratinization, androgens, sebum, Propionibacterium acnes, and inflammation. Generation of inflammation in acne lesions is a complex process. Potential instigators of inflammation may be derived from the breakdown products of sebaceous lipids, derived from P. acnes, or from various immunologic mechanisms directed against this organism (6).
Comedones exhibit hyperkeratinization, and the keratin is bound more avidly as a result of increased levels of intercellular adhesion materials (7). Ultrastructurally, follicular keratinocytes in comedones possess increased numbers of desmosomes and tonofilaments, which contribute to hypercornification (8). The black color of open comedones (blackheads) appears to be related to densely packed keratinocytes, bacteria, and bacterial products located at the surface. If dilation of the follicular orifice does not occur, continued thinning of the infundibular wall and ultimate rupture of the closed comedone becomes more probable.
There is substantial evidence linking the activation of sebum production to androgens, specifically testosterone and dihydrotestosterone (DHT) (9). Circulating testosterone is converted in tissues to the more potent DHT by the enzyme 5α-reductase. The type 1 isoform of 5α-reductase is located primarily in sebocytes, but is also found in the epidermis, infundibular hair follicle keratinocytes (10), dermal papilla cells, sweat glands, and fibroblasts. Activity of the type 1 isoform of 5α-reductase was greater in sebaceous glands from acne-prone areas of the skin than in those from non–acne-prone areas, suggesting that regional differences in pilosebaceous production of DHT may play a role in acne (11).
The majority of acne patients, especially male patients, do not show abnormal serum androgen levels, though tissue androgen levels may be elevated (12). One study found significantly higher mean serum androgen levels in females with acne than in those without acne, although values were in the range of normal; in addition, sebaceous glands from the women with acne showed higher mean 5α-reductase type 1 activity, although the difference was not statistically significant (13). This study suggests that women with acne may have higher serum androgen levels and perhaps a greater capacity to produce DHT within sebaceous glands.
Sebum production in acne is elevated and appears to correlate with disease severity. The beneficial effect of isotretinoin (13-cis-retinoic acid) appears to result largely from reduction in sebum production, and after 2 months of treatment, a 70% reduction in sebum excretion is achieved (14). These changes are associated histologically with a marked reduction in sebaceous gland size.
Of all the microorganisms identified in the follicular infundibulum, only P. acnes appears to be consistently involved in the pathogenesis of acne lesions (15). Nevertheless, levels of this organism may not be consistently greater in lesional skin than in normal skin (16). Immunologic reactivity to this organism may contribute to the inflammation in acne lesions.
Differential Diagnosis. When a hair follicle or follicle-derived keratin is not evident on the biopsy and the sections show only inflammation with neutrophils and histiocytes, an infectious etiology could be considered in the appropriate clinical context.
Principles of Management. Topical retinoids are a mainstay of treatment of acne vulgaris, targeting precursor microcomedones (17). Topical benzoyl peroxide also has comedolytic and antibacterial properties, and is often used in combination with topical antibiotics. Oral antibiotics in the tetracycline class are often added for their anti-inflammatory properties when erythematous papules and pustules are present. In severe acne with nodulocystic lesions or scarring, oral isotretinoin may be required for adequate control (18).
Steroid Acne
Clinical Summary. Steroid acne is a folliculitis caused by the use of corticosteroids, including systemic, topical, inhaled (19), and intranasal (20) steroids. Despite its well-known existence, steroid acne has become more prevalent with the creation of increasingly potent topical preparations as well as various treatment regimens for cancer and organ transplantation (21). Although other medications have been implicated in acneiform eruptions, steroid acne results in a distinctive clinical picture characterized by the sudden appearance of monomorphous papulopustules predominantly on the upper trunk and arms but also on the face (22). Comedones are not apparent. The underlying rash for which topical steroids are used initially improves, followed by exacerbation of the rash (23). The exacerbation is controlled only by continued use of topical steroids (physical dependence). Resolution typically occurs without scarring. Perioral dermatitis may be caused by topical corticosteroids (see the discussion on Perioral Dermatitis).
Histopathology. Acne due to both topical and systemic corticosteroids shows similar histologic features. Despite the apparent absence of comedones clinically, Hurwitz described histologic features that resembled those of acne vulgaris (Fig. 18-4), albeit with an accelerated rate of development (21). In chronologic order, biopsied flesh-colored papules that measured 1 mm showed infundibular spongiosis, hyperkeratosis, perifollicular edema, microcomedo formation, and infundibular wall thinning, sometimes with infundibular rupture. It was more common, however, to see only infundibular dilation with compact hyperkeratosis. Biopsied lesions that were 2 mm or greater and clinically inflamed frequently showed infundibular rupture, necrotic keratinocytes, and surrounding suppurative and granulomatous inflammation that included multinucleated giant cells amid keratinous debris. Dilated blood vessels were also seen.
Figure 18-4 Steroid acne. Pustules in steroid acne are monomorphous and are indistinguishable from acne vulgaris.
Pathogenesis. Although the exact pathogenesis is not known, three phases of lesion development have been described as a result of “addiction” to topical steroids (23): (a) initial improvement of the rash is due to the anti-inflammatory properties of topical corticosteroids, (b) local immunosuppressive effects of the steroids result in bacterial overgrowth, and (c) on withdrawal of steroids, there is rebound and flaring secondary to bacterial overgrowth. In addition to the chronologic sequence described under Histopathology, some authors have described a pathologic process that is the reverse of acne vulgaris, beginning with folliculitis, which proceeds to rupture and concludes with comedone formation (a so-called secondary comedone) (24). In a study of steroid acne due to systemic therapy, 80% of 125 patients showed significant numbers of Pityrosporum ovale in lesional follicles, and itraconazole, an oral antifungal agent, proved more efficacious than other medications (25).
Differential Diagnosis. The differential diagnosis would include acne vulgaris and other types of acute folliculitis.
Principles of Management. Withdrawal of the oral or topical corticosteroids that caused the eruption eventually results in abatement of lesions, though a rebound flare is often the norm and may require tapering of steroids. Treatment modalities used in acne vulgaris can sometimes hasten resolution.
Perioral Dermatitis
Clinical Summary. Recognition of this relatively common facial eruption is important because of its resemblance to rosacea, seborrheic dermatitis, and occasionally lupus erythematosus. Predominantly affecting white women of European extraction ranging in age from the midteens into middle age, it results in fine, follicular-based perioral papules and, more rarely, in a periocular distribution (periocular dermatitis) (26). The papules are single, grouped, or confluent, with minimal scaling. Pinhead-sized pustules may occur in more severe cases. There is sparing of a 5-mm zone around the vermilion border (22). The condition lacks significant telangiectasia. It is commonly associated with topically applied preparations, especially topical corticosteroids and cosmetics, as well as inhaled and intranasal steroids (27). Perioral dermatitis in children occurs primarily as a result of topical steroid use and is almost identical to the disease in adults (28).
Childhood granulomatous periorificial dermatitis is an acneiform facial eruption that shows some similarity to granulomatous rosacea and perioral dermatitis in children but does have some distinctive features (29,30). The current terminology was first coined in 1989 (29), although other terms have been proposed, such as facial Afro-Caribbean childhood eruption, reflecting its usual incidence on the face of black children (31,32). Other features that distinguish childhood granulomatous periorificial dermatitis from childhood perioral dermatitis and acne vulgaris include (a) exclusive incidence in healthy prepubertal children and (b) absence of pustules and lack of circumoral sparing. Clinical lesions may be flesh-colored, hypopigmented, or reddish yellow; are small (1 to 3 mm); monomorphous; and occur periorally, perinasally, and periorbitally. Lesions are asymptomatic and self-limited but may last for years, typically healing without residual except for some small, pitted scars in a small number of patients (33). Cases with extrafacial and generalized lesions have been reported (33).
Histopathology. Some authors consider perioral dermatitis to be a variant of rosacea, with indistinguishable histologic features (34,35), whereas others consider the two entities to be distinct (36,37), with some histologic overlap. A proposed clinical classification scheme for rosacea published in 2002 did not classify steroid-induced acneiform eruptions or perioral dermatitis as rosacea variants, citing insufficient evidence (37).
The following histologic characteristics of perioral dermatitis have been described (36). Fully developed lesions show spongiosis of the follicular infundibulum with mild mononuclear cell exocytosis (Fig. 18-5), with similar changes sometimes observed in the epidermis adjacent to the involved follicle. The epidermis may show mild acanthosis and parakeratosis, particularly about follicular ostia. The perifollicular dermis shows lymphohistiocytic inflammation around vessels, and in rare cases plasma cells may be prominent. Less developed lesions may exhibit only dermal inflammation. Lesions tend to lack the dermal edema and telangiectasias characteristic of rosacea and show more noticeable epidermal changes. Acute folliculitis is uncommon.
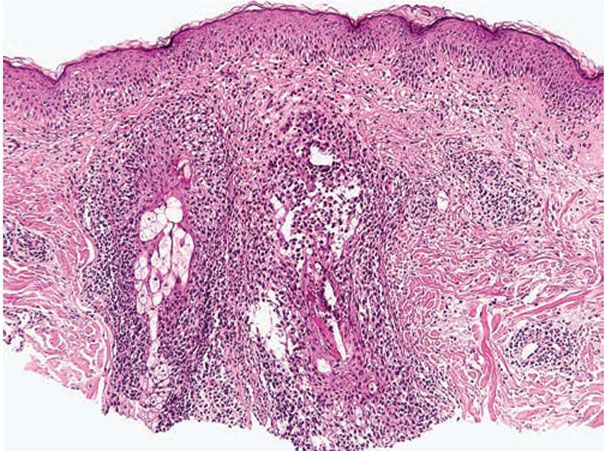
Figure 18-5 Perioral dermatitis. There is perifollicular mononuclear inflammation with infundibular spongiosis and exocytosis of lymphocytes.
Lesions of childhood granulomatous periorificial dermatitis often resemble granulomatous rosacea, with noncaseating perifollicular granulomas with some giant cells (29,33). Other features include epidermal changes consisting of mild hyperkeratosis and spongiosis, as well as dermal inflammation, composed of lymphocytes and histiocytes, that is mild to moderate, perivascular, and perifollicular. The granulomatous component is variable; some biopsies lacked granuloma formation or follicular involvement altogether. Some cases showed focal follicular rupture with inflammation to the released contents (29). Special stains for fungi and mycobacteria have been negative (33).
Pathogenesis. Many possible etiologies for perioral dermatitis have been proposed, including Candida organisms, bacteria, particularly of the fusiform type, Demodex mites, a wide range of contactants, both irritant and allergic (including cosmetics and fluoride-containing toothpaste), hormones (including birth control pills), topical corticosteroids, and emotional as well as systemic conditions. An Australian survey found an increased risk of developing perioral dermatitis with use of foundation makeup along with moisturizer and night cream (38).
The etiology of childhood granulomatous periorificial dermatitis is not known, but an external contactant has been implicated in some cases, with various agents, including topical fluorinated corticosteroids, reported (33).
Differential Diagnosis. The histology may resemble rosacea, though the dermal edema and telangiectasias characteristic of rosacea are often lacking.
Principles of Management. Identification and avoidance of inciting factors, especially topical products, is foremost. Topical and oral antibiotics, particularly the tetracyclines, can also be beneficial.
Rosacea
Clinical Summary. Rosacea is an acneiform inflammatory condition that primarily affects the face and is more common after the age of 30 years (39). It is characterized by background erythema within which scattered telangiectasias, papules, and occasional pustules develop (Fig. 18-6). It typically affects the nose, cheeks, glabella, and chin, and it is usually bilateral but occasionally unilateral or focal. If it is severe, lesions may spread to the neck and, rarely, become disseminated. Rhinophyma, a bulbous swelling of the soft tissue of the nose, is a late complication that occurs almost exclusively in men. Eye changes—especially blepharitis and conjunctivitis—are quite common in rosacea, and in 5% of cases a painful keratitis can develop (40). Rosacea can precipitate persistent facial lymphedema.
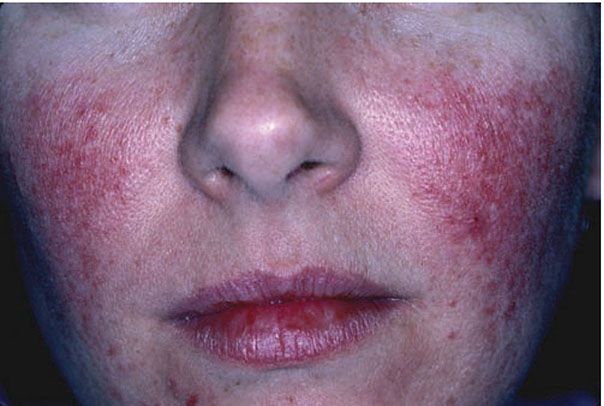
Figure 18-6 Rosacea. Scattered erythematous papules with background telangiectasias that give the face a “ruddy” appearance.
A recently proposed clinical classification scheme for rosacea delineated four subtypes: (a) erythematotelangiectatic, (b) papulopustular, (c) phymatous, and (d) ocular (37,41).
Histopathology. Vascular dilation of upper and mid-dermal vessels with perivascular and perifollicular lymphohistiocytic inflammation (and occasional plasma cells) is generally present in all cases (Fig. 18-7). Lymphatic dilation is also common and may be prominent. With mild follicular involvement, there is infundibular spongiosis and lymphocyte exocytosis. With more extensive follicular involvement, neutrophils accumulate, resulting in a superficial pustule. Reflecting the clinical presentation, various other pathologic changes can be seen from case to case, including organization of dermal lymphocytes into small nodular aggregates (42).
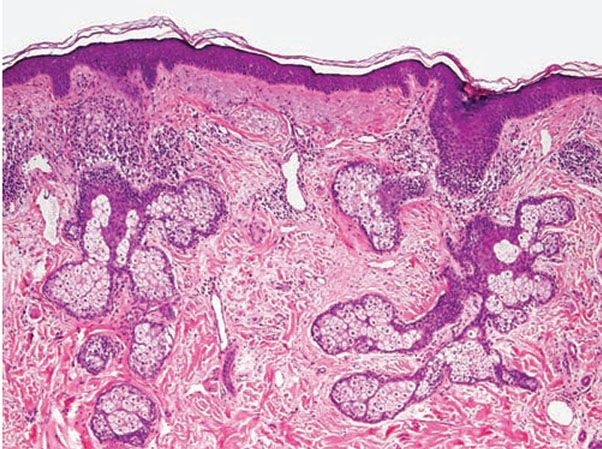
Figure 18-7 Rosacea. Patchy lymphocytic inflammation surrounds vellus hair follicles as well as some blood vessels. Telangiectasias and solar elastosis are present.
Granulomatous infiltrates are reported to occur in about 10% of all cases of rosacea (42) (Fig. 18-8), and caseation necrosis has been identified in about 10% of these patients (43). Granulomatous rosacea can histologically mimic mycobacterial infections, with epithelioid histiocytes forming a tuberculoid pattern (also see discussion on lupus miliaris disseminatus facei). In such cases, other investigative procedures, including tissue cultures, may be necessary. Less frequently, the changes resemble cutaneous sarcoidosis. Multinucleated giant cells of the foreign-body type may aggregate around follicular contents spilled from a ruptured follicle.

Figure 18-8 Granulomatous rosacea. The infiltrate is dense and has a prominent histiocytic component, clustered into granulomas. Telangiectatic blood vessels are present.
In a study of rhinophyma, the classic or common type showed histologic features of fully developed rosacea, except for sebaceous gland hyperplasia, which can be prominent (44). The sebaceous ducts become dilated and filled with keratin and sebum. The same study showed that in patients with the severe form of rhinophyma, there is marked dermal thickening with sclerotic collagen bundles and large amounts of mucin, an absence of pilosebaceous structures, sparse inflammation, and telangiectasias (44). Many spindle-shaped and “bizarre” cells staining for factor XIIIa were seen in the interstitium. It was noted that the microscopic findings in the severe form of rhinophyma demonstrate many similarities to elephantiasis nostras caused by chronic lower-extremity lymphedema.
Pathogenesis. Although triggers of rosacea are well known, the pathogenesis of the disease is unknown. It is probable that the etiology of rosacea is multifactorial, but sun damage appears to be a constant feature. Rosacea is not associated with an increase in sebum excretion. Dahl proposed that the vascular dilation leads to dermal edema, which in turn promotes the inflammation that is ultimately responsible for the sequelae of rosacea (45). In this model, warm facial skin from increased blood flow may modify the behavior of bacteria and/or Demodex or change the enzymatic activity of keratinocytes or other skin cells, leading to altered metabolism and inciting inflammation.
The role of Demodex organisms has been debated for decades. Some reports claimed a significant association of mites in rosacea biopsies (46,47), but other studies did not support this claim (48). Although a definitive causative role for Demodex in rosacea is uncertain, the mites may represent an important cofactor in heightening disease severity (47).
An association with gastrointestinal Helicobacter pylori infection has been the subject of investigation. In one study, a number of rosacea patients had gastrointestinal symptoms related to gastritis; the prevalence of H. pylori in those patients with rosacea was 88%, compared to 65% in the control group without rosacea (but with nonulcer dyspepsia) (49). Furthermore, eradication of H. pylori with 1 week of therapy that included oral metronidazole, omeprazole, clarithromycin, and topical intraoral metronidazole resulted in marked improvement or resolution of rosacea symptoms after 2 to 4 weeks (50). Attempts to find H. pylori in the skin have been unsuccessful. A proposed pathogenesis is increased flushing due to vasodilators such as nitric oxide and gastrin, and inflammatory cytokines such as tissue necrosis factor-α and interleukin-8 (IL-8), all produced by H. pylori (51). Nevertheless, definitive studies validating an association of H. pylori with rosacea have yet to be done.
Differential Diagnosis. Perioral dermatitis is histologically similar. In lesions with a prominent granulomatous component, infection can enter the differential diagnosis.
Principles of Management. Avoidance of triggers that incite flushing, which invariably includes sunlight, is a staple of management. Topical antibiotics, sulfa products, and azelaic acid may be used (52). Tetracycline antibiotics are often required for papulopustular lesions.
Demodicidosis
Clinical Summary. Demodicidosis is the term for cutaneous disease caused by mites of the genus Demodex. Despite more than 65 years of investigation into the role of Demodex mites in human skin disease, questions remain. Aside from their controversial role in rosacea, Demodex mites have been implicated in follicular-based skin eruptions resembling rosacea, albeit with some unique features that warrant consideration as distinct entities (53). There are at least three clinical forms of demodicidosis (53). The first, pityriasis folliculorum, is characterized by facial erythema with fine follicular plugs and scale producing a “nutmeg-grater” or “sandpaper-like” appearance. It usually affects women and may be associated with itching and burning. The second, rosacea-like demodicidosis, resembles rosacea, in that there are papules and pustules. In contrast to rosacea, however, there is follicular scaling, sudden onset, rapid progression, and no history of flushing. There may be eyelid involvement (demodectic blepharitis) and poor general health, such as diabetes mellitus (53). Lastly, demodicidosis gravis resembles severe granulomatous rosacea. These forms may represent a spectrum of disease in which the clinical expression depends on the degree and duration of Demodex infestation and the individual’s age and overall health (53). Demodex may also be important in the pathogenesis of nonclassic presentations involving facial pruritus and erythema and nonspecific acneiform lesions (54).
Demodex are obligatory parasites frequently found in mammalian pilosebaceous units, and colonization is usually asymptomatic (55). The mites found in humans are of the species Demodex folliculorum and D. brevis. D. folliculorum, the predominant mite, has a longer body, inhabits the infundibulum of hair follicles, and is found almost exclusively on the face (56,57). D. brevis is smaller and lives exclusively within sebaceous glands on the face and trunk (56,57).
Demodicidosis has also been classified as primary or secondary, with the latter occurring on diseased skin (58). The primary form affects nondiseased skin, is caused by D. folliculorum, and causes an eruption in the T-zone of the face, involving 8% to 15% of the face. Secondary demodicidosis is characterized by a symmetric eruption of the malar region, covering 30% to 40% of the face, and is caused by D. brevis. There are other distinguishing features, such as onset of erythema, pruritus, and seasonality (58).
A study of consecutive biopsies submitted to one dermatopathology laboratory showed Demodex mites in 10% of all biopsies and 12% of all follicles (56). Their prevalence increased with age, and males were more heavily infested. In another study, there was a nonrandom association between histologic folliculitis and presence of Demodex in the follicles (57); mites were found in 42% of inflamed follicles but in only 10% of noninflamed follicles, whereas 83% of follicles containing Demodex showed inflammation. This demonstrated a strong association but not a cause-and-effect relation because the possibility that Demodex could preferentially select inflamed follicles was raised. Additional support for a pathogenic role was provided by reports of sudden facial eruptions in which numerous Demodex mites were identified and the eruption failed to respond to therapy for rosacea but cleared quickly with treatments directed against Demodex, such as crotamiton or ivermectin and permethrin (59).
Some believe that Demodex mites are pathogenic only in large numbers (59), but an individual’s overall health also appears to be important, as demonstrated by reports in immunocompromised children (60) and multiple reports in patients with HIV and AIDS, in which the eruption may also involve the neck, upper trunk, and extremities (61). One study stated that demodicidosis is prevalent even in immunocompetent individuals (46), and it has been reported in immunocompetent children (62). There is a coassociation with immune response, including subtypes and functional activity of lymphocytes, and circulating levels of immune complexes (63).
Human demodectic alopecia may be a real entity characterized by alopecia, erythema, and scale, with numerous Demodex mites in affected follicles (64). It resembles canine demodectic mange and has been successfully treated with permethrin. Demodex may also cause a refractory scalp folliculitis (65).
Histopathology. The diagnosis can be made in the clinical setting by examining scale with 40% KOH (66) or by standardized skin surface biopsy (SSSB), in which cyanoacrylate glue is used to sample the horny layer and follicular contents (67). The presence of five or more mites in a single low-power field by KOH or more than 5/cm2 by SSSB is considered significant.
Biopsies of pityriasis folliculorum show a perivascular and diffuse dermal lymphocytic infiltrate without granuloma formation (53). Excess numbers of Demodex mites are present within the infundibulum of pilosebaceous units, and may be associated with an infundibular pustule. Biopsies of rosacea-like demodicidosis (Fig. 18-9) show a primarily perifollicular lymphohistiocytic infiltrate, often with neutrophils or granulomatous inflammation containing multinucleated giant cells (53,68). Mites may be present in the dermis amid the inflammation. Demodicidosis gravis shows granulomas with central necrosis (caseation) and foreign-body-type multinucleated giant cells.
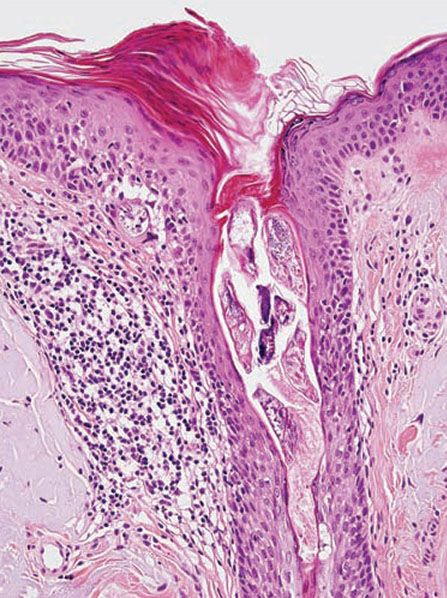
Figure 18-9 Demodicidosis. The follicular infundibulum contains multiple Demodex mites, and there is perifollicular lymphohistiocytic inflammation. Where the left side of the follicular epithelium meets the epidermis, an organism appears to be perforating through to the dermis.
Pathogenesis. The pathogenesis of Demodex-related disease may be linked to one of the following: (a) blockage of hair follicles and sebaceous ducts due to reactive epithelial hyperplasia and hyperkeratinization, (b) mites serving as vectors for bacteria, (c) a foreign-body reaction to the mite, or (d) induction of host immunity by the mites and their waste (55). Some mite antigens may also elicit a delayed hypersensitivity reaction (47). One paper suggested that a mite-derived lipase could potentially release fatty acids from serum triglycerides, producing an irritant reaction (69).
Differential Diagnosis. Biopsies of rosacea will commonly demonstrate organisms. The problem in Demodex-related skin disease is determining whether the organisms are truly pathogenic, since they are frequently seen within hair follicles, especially on the head.
Principles of Management. The mite population can be kept in check by cleansing the face regularly, by using crotamiton or permethrin cream, or oral ivermectin or metronidazole.
Lupus Miliaris Disseminatus Faciei
Clinical Summary. Lupus miliaris disseminatus faciei (acne agminata) is considered by some to be a granulomatous variant of rosacea, but other authors consider it to be a distinctive rosacea-like syndrome (70). The clinical presentation is distinctive, with discrete, flesh-colored or mildly erythematous papules—arranged singly or in small groups—involving the eyelids and upper lip, areas where rosacea lesions are not commonly found (71,72). The background erythema and telangiectasias of rosacea are lacking. Papules frequently persist for 12 to 24 months and are resistant to standard rosacea therapy, although spontaneous healing can occur. Unusual presentations have included axillary lesions (73) and during pregnancy in a patient with cutaneous lupus erythematosus (74). One group proposed the use of equally cumbersome terminology, “facial idiopathic granulomas with regressive evolution” (F.I.GU.R.E.) (75).
Histopathology. Biopsy specimens sectioned through the central portion of a papular lesion demonstrate one of the most highly characteristic patterns of cutaneous histopathology. Surrounding a usually large area of caseous necrosis, aggregates of epithelioid histiocytes and occasional multinucleate giant cells form a “tubercle” with sparse lymphoid inflammation at the periphery (Fig. 18-10).
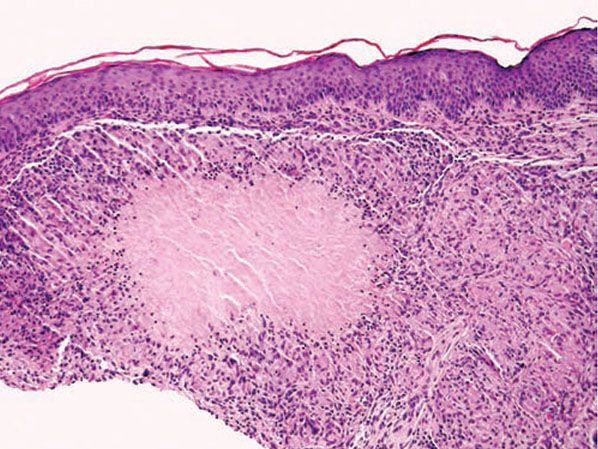
Figure 18-10 Lupus miliaris disseminatus faciei. Caseous necrosis is surrounded by epithelioid histiocytes and peripheral lymphocytes, forming a “tubercle” that mimics Mycobacterium tuberculosis infection.
In one study, this characteristic histology was only found in a minority of patients in fully developed lesions, and an attempt was made to describe the histologic features of early, fully developed, and late lesions (76,77). Early, developing lesions show perivascular and perifollicular lymphocytes and some histiocytes. Fully developed lesions show a sarcoidal granuloma (first stage), sometimes accompanied by an abscess (second stage) or surrounding an area of caseation necrosis (third stage). The granuloma may be perifollicular and involve one or more hairs, and the follicle may be “ruptured” with a neutrophilic response. Granulomas may also be compact or loose and may occupy the upper or lower dermis, and some hug the epidermis. Late lesions demonstrate scattered lymphocytes, histiocytes, and neutrophils and perifollicular fibrosis. Additional findings include follicular dilation, hyperkeratosis, sometimes combined with follicular plugging, and pigment incontinence (76).
Despite the histologic picture, no direct relationship with tuberculosis has been documented, including testing using polymerase chain reaction with DNA fragments specific for Mycobacterium tuberculosis complex (78).
Differential Diagnosis. The histology may be indistinguishable from cutaneous tuberculosis, a rare entity.
Principles of Management. Difficult to control, spontaneous resolution may occur after a couple of years. Tetracycline antibiotics are a mainstay of treatment, but there are several case reports describing other therapies in refractory cases (79).
Eosinophilic Pustular Folliculitis
Clinical Summary. Ofuji originally described eosinophilic pustular folliculitis (EPF) in immunocompetent Japanese patients as itchy follicular papules and pustules arranged in arcuate plaques with central healing and peripheral spread (80–82). Although most reported cases have been from Japan, the condition is now known to be geographically more widespread. Lesions typically involve seborrheic areas, including the face, trunk, and upper arms, with documented reports of extrafollicular lesions of the palms and soles (83). Moderate leukocytosis and eosinophilia in the peripheral blood may be present. There is usually spontaneous healing over months to years. There are reports of the eruption in children (84), including neonates (85), and in this subset lesions occur predominantly on the scalp. Scarring alopecia of the scalp in an adult has been reported (86). Although a cause has not been identified, rare medication-induced cases have been described (87). Cases have been associated with nevoid basal cell carcinoma syndrome (88), hepatitis C infection (89), and infestations (90).
Lesions with similar histology are well documented in patients with HIV infection (91) and other immunocompromised conditions, such as myelodysplastic syndrome (92), non-Hodgkin lymphoma (93), B-cell chronic lymphatic leukemia (94), polycythemia vera (95), after bone marrow transplantation (96), and after autologous peripheral blood stem cell transplantation (97). There appears to be enough clinical dissimilarity from the disorder described by Ofuji to consider HIV-associated disease a distinct entity, and use of the terms eosinophilic folliculitis or HIV-associated eosinophilic folliculitis was recommended to reflect this (91). Another classification scheme proposed the designations classical, infancy associated, and immunosuppression associated (mostly HIV) (81). In HIV patients, lesions tend to be urticarial papules with less of a tendency to become pustular, and leukocytosis is less common (98). They are most commonly found on the face, scalp, and upper trunk and often are excoriated.
Histopathology. In Ofuji disease, there is exocytosis of eosinophils into a spongiotic follicular infundibulum and accompanying sebaceous gland, eventually forming eosinophilic micropustules (Fig. 18-11) (86,99). The epidermis adjacent to the affected follicle may contain lymphocytes and eosinophils, with the latter aggregating into small eosinophilic pustules that are subcorneal or intraepidermal; these epidermal changes reflect the histologic picture seen in palmoplantar lesions, where follicles are absent. In more inflamed lesions, neutrophils may be present. In the dermis, there are perivascular and interstitial infiltrates of lymphocytes and numerous eosinophils, which may surround the sweat glands. Associated follicular mucinosis has been reported (100).
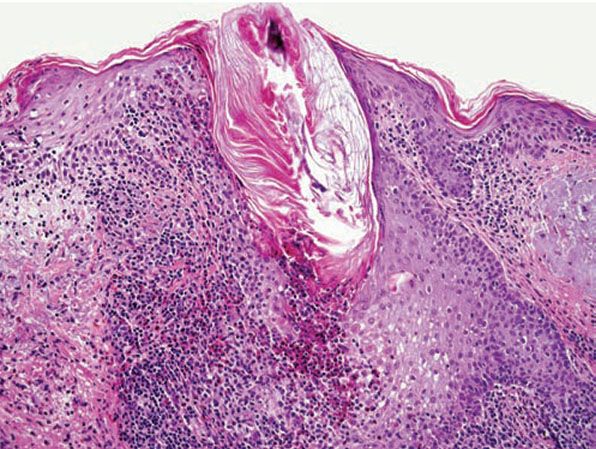
Figure 18-11 Eosinophilic pustular folliculitis. Eosinophils and lymphocytes surround and infiltrate the follicle, forming intrafollicular eosinophilic micropustules.
A study of 52 biopsies in 50 HIV-positive patients best described the histology of HIV-associated eosinophilic folliculitis (98). Perifollicular and intrafollicular lymphocytes and eosinophils are concentrated about the isthmus, and may involve the sebaceous duct. There is spongiosis of the follicular epithelium. Eosinophils and lymphocytes may be seen aggregating in the hair canal, but neutrophils are rare. In early lesions, lymphocytes may predominate and be distributed perifollicularly and interstitially. In more developed lesions, dermal inflammation diminishes and perifollicular/follicular inflammation increases. Less common findings include inflammation of the sebaceous gland, eosinophilic pustules, and follicular rupture (98). Dense eosinophilic infiltrates with degranulation and flame figures, resembling Wells syndrome, may sometimes be seen. A small number of macrophages can be present. Bacteria, yeast, or Demodex may be identified, albeit away from the areas of inflammation. Because the disorder is highly pruritic, excoriation is a common secondary finding.
Features that may help to distinguish suppurative folliculitis in HIV-positive patients from HIV-associated eosinophilic folliculitis have been described; suppurative folliculitis in HIV-positive patients more commonly shows an infiltrate dominated by neutrophils and macrophages, the presence of microorganisms amid the inflammation, and rupture of the involved follicle. The use of transverse histologic sections has been advocated over vertical sections for increasing diagnostic sensitivity and demonstrating the key pathologic findings in HIV-associated eosinophilic folliculitis (101).
Pathogenesis. The cause of Ofuji disease (EPF) is not known. Although the prevalence in Japan could not be explained by HLA patterns (86), the occurrence in brothers in the neonatal period hints at some inherited predisposition or possibly an infectious etiology (102).
The perivascular infiltrating cells in EPF were found to be mostly T lymphocytes, with some CD68+ myelomonocytic cells, and most of the eosinophils were positive for eosinophil cationic protein (103). A moderate increase in the number of tryptase-positive, chymase-negative mast cells, the type predominantly found in lung and small intestine, was noted around hair follicles and sebaceous glands (99).
In another study of three patients with EPF, serum levels of interferon-γ and IL-2, -4, and -6 were measured before and after successful treatment with indomethacin (104). Investigators found elevated IL-4 in the EPF patients, which remained unchanged after treatment, and increased interferon-γ with disease remission.
Ultrastructurally, in EPF lesions characterized by an infundibular pustule, acantholytic outer root sheath keratinocytes showed desmosomal cleavage with microvilli formation, and some contained sebaceous lipid droplets (105). Apposition of T lymphocytes and Langerhans cells was seen.
Differential Diagnosis. In the differential diagnosis, eosinophilic follicular pustules may be seen in erythema toxicum neonatorum, and intraepidermal eosinophilic vesicles can be seen both in acropustulosis and the vesicular phase of incontinentia pigmenti. However, the clinical presentation of these conditions would generally be significantly different than that in EPF, although it has been suggested that EPF in infants and infantile acropustulosis may be variants of the same disorder (106). Identical eosinophilic pustular eruptions have been described in association with fungal infections due to dermatophytes (107). A folliculocentric arthropod bite can sometimes enter into the differential diagnosis.
Principles of Management. Ofuji’s eosinophilic pustular folliculitis is managed primarily with systemic steroids or dapsone. Treatments for HIV-associated eosinophilic folliculitis include topical steroids, antihistamines, phototherapy, itraconazole, topical permethrin, and isotretinoin (22).
Follicular Mucinosis and Alopecia Mucinosa
Clinical Summary. Follicular mucinosis is characterized clinically by grouped erythematous papules and/or plaques that may be markedly indurated or nodular and histologically by mucin accumulation in hair follicles (Fig. 18-12) (108). It can be classified into two types: a primary (idiopathic) type and a secondary variety. The primary form tends to have a shorter but benign course. The secondary type has been associated with numerous benign and malignant conditions, including lymphomas, of which the majority are mycosis fungoides. A distinct variant of mycosis fungoides—follicular mycosis fungoides—may or may not be associated with follicular mucinosis (109,110).
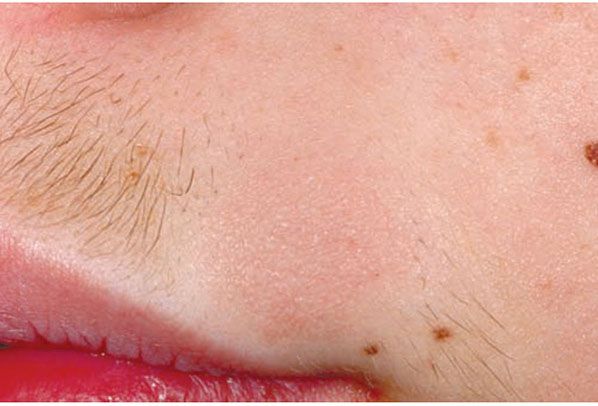
Figure 18-12 Follicular mucinosis/alopecia mucinosa. This round, slightly elevated pink plaque in an adolescent boy has resulted in clinically apparent alopecia because the hairs at this site are coarser.
The primary form tends to affect children and young adults more frequently and resolves spontaneously in several months (acute benign type) or several years (chronic benign type). It is often confined to the head and neck but may be disseminated. The secondary type tends to form more widespread plaques and is almost always a disorder of adults.
The secondary type has been found in association with other lymphoproliferative disorders, including Hodgkin disease (111,112), cutaneous B-cell lymphoma (113), acute myeloblastic leukemia (114), chronic lymphocytic leukemia (112), and syringolymphoid hyperplasia with cutaneous T-cell lymphoma (115), as well as a number of inflammatory cutaneous disorders such as chronic discoid lupus erythematosus (116), angiolymphoid hyperplasia, alopecia areata (AA) (117), eosinophilic pustular folliculitis (100), spongiotic dermatitis, lichen striatus, arthropod bites, sarcoidosis, Goodpasture syndrome (118), leprosy (119), and growths such as verrucae (118), melanocytic nevi, and squamous cell carcinoma of the tongue (120). The numerous associations with production of follicular mucin certainly suggest that this is a relatively nonspecific reaction pattern.
There has been controversy about whether the histopathology allows for distinction between the primary and secondary forms. Initially it was considered that the histopathology could be predictive (121). Later, some researchers claimed that transition from the benign form to a lymphomatous type could occur (122), whereas others disputed this finding (118). In 1989, a study of 59 cases concluded that there was no clinical or pathologic pattern by which the ultimate outcome of the condition could be predicted (111).
Others have reported that adults older than 40 years with widespread follicular mucinosis are at increased risk for mycosis fungoides or Sézary syndrome (118), although Cerroni et al. (122) claimed that the criteria purported to differentiate lymphoma-associated follicular mucinosis from the idiopathic type were not effective. A long-term follow-up study (median 10 years) of seven patients younger than 40 years with primary follicular mucinosis failed to demonstrate progression to cutaneous T-cell lymphoma, despite the presence of a T-cell clone in five of the patients (123).
In 1957, Pinkus (124) described alopecia mucinosa, the term used when follicular mucinosis affects terminal hair-bearing areas and is associated with hair loss (Fig. 18-13). Papules and plaques may be present or inconspicuous in this form, which may show only alopecia. Scarring is seen more commonly when alopecia mucinosa is associated with cutaneous T-cell lymphoma. Others claim that alopecia mucinosa is simply one of the many morphologic variants of mycosis fungoides (125), whereas LeBoit (126) acknowledged the paradoxes of alopecia mucinosa that have led to the debate over classification.
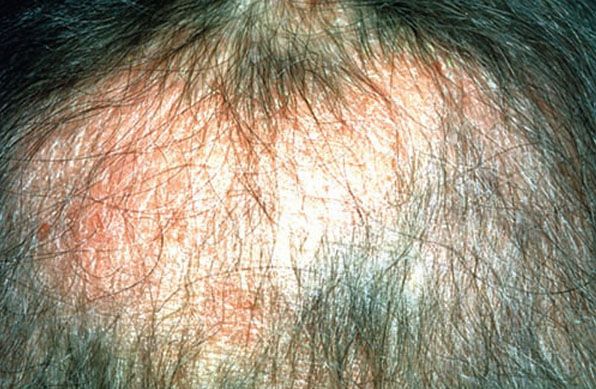
Figure 18-13 Alopecia mucinosa. Diffuse thinning of the scalp associated with slightly elevated, scaly plaques. The scalp erythema and poorly defined areas of alopecia distinguish this example from alopecia areata.
Histopathology. Within the outer root sheath and sebaceous gland epithelium, there is reticular epithelial degeneration that sometimes evolves into more extensive cavitation within which mucin is deposited (Fig. 18-14). Occasionally, little mucin can be detected, perhaps because of removal of this water-soluble material in the processing procedure. The deposited mucin is an acid mucopolysaccharide that stains metachromatically with toluidine blue at pH 3.0, as well as with Alcian blue at acid pH. The fact that it can be substantially removed by digestion with hyaluronidase demonstrates that the mucin is predominantly hyaluronic acid. Colloidal iron stain may also be used for its detection.

Figure 18-14 Follicular mucinosis. The outer root sheath epithelium of several follicles shows reticular degeneration with areas of cavitation. There are intercellular strands of bluish-staining mucin.
Inflammation is perivascular and perifollicular, and composed of lymphocytes and histiocytes, but there can also be eosinophils. There may be exocytosis into the outer root sheath epithelium of the infundibulum and the sebaceous gland epithelium. Although individual pathologic criteria are not absolutely diagnostic of the type of follicular mucinosis (primary or secondary), features that have been proposed as favoring a lymphoma-associated lesion include an increased density of the perifollicular infiltrate with substantial folliculotropism, a dense bandlike infiltrate containing atypical lymphocytes, epidermotropism, a predominance of CD4+ lymphocytes (vs. equal numbers of CD4 and CD8 cells), and a clonal gene rearrangement (Fig. 18-15) (127,128).
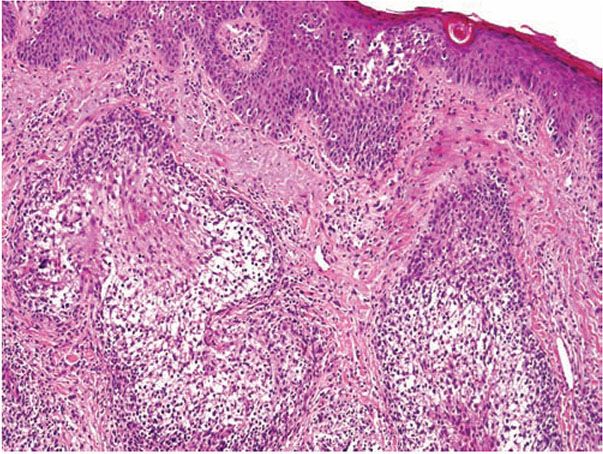
Figure 18-15 Follicular mucinosis associated with folliculotropic mycosis fungoides. In addition to changes of follicular mucinosis, there is extensive folliculotropism of lymphocytes. Epidermotropism of large lymphocytes is also present.
Pathogenesis. Electron microscopic studies have shown that the mucin is a product of the outer root sheath epithelial cells. The cytoplasm shows prominent, dilated, rough-surfaced endoplasmic reticulum containing fine, granular, filamentous material that is secreted into the intercellular spaces (129).
Differential Diagnosis. Determining whether follicular mucinosis is primary or an incidental, secondary finding is a diagnostic challenge. Correlation with the clinical presentation is crucial.
Principles of Management. While primary follicular mucinosis may spontaneously resolve, topical or systemic steroids as a first-line treatment can help. In treating secondary follicular mucinosis, therapy is geared toward the associated condition.
Keratosis Pilaris
Clinical Summary. This common persistent condition characteristically affects the lateral aspect of the arms, thighs, and buttocks. Keratotic follicular papules, sometimes with surrounding erythema, are present. They are usually asymptomatic. Keratosis pilaris may be seen in association with ichthyosis vulgaris (130), and appears to be more common in patients with atopic dermatitis.
Similar lesions may form as part of a variety of more extensive erythematous keratinizing disorders. Keratosis pilaris rubra is a variant that shows more widespread skin involvement and more prominent erythema (131). Keratosis pilaris atrophicans represents a spectrum of clinical conditions, including keratosis follicularis spinulosa decalvans (132,133), in which the involved follicle becomes atrophic and destroyed, sometimes associated with scarring alopecia of the scalp.
Histopathology. An orthokeratotic keratin plug blocks and dilates the orifice and upper portion of the follicular infundibulum (Fig. 18-16). A twisted hair shaft may be trapped within this keratin material, and a mild perivascular mononuclear cell infiltrate is usually present in the adjacent dermis.
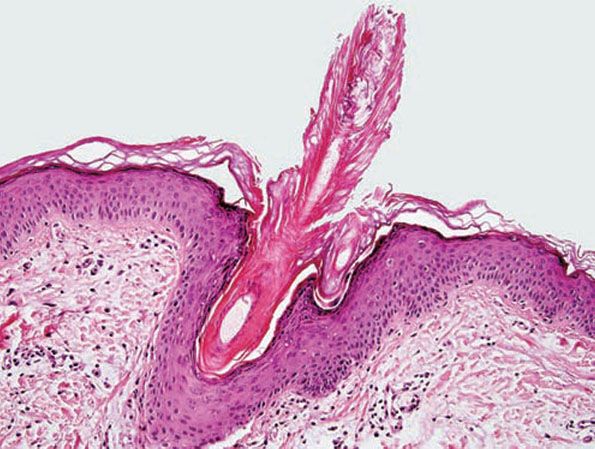
Figure 18-16 Keratosis pilaris. The follicle is plugged with compact orthokeratin that protrudes above the skin surface.
Pathogenesis. Genetic factors may play a role in this condition. Patients with a generalized form of keratosis pilaris were found to have a chromosome 18p deletion, implicating a gene on the short arm of chromosome 18 (134).
Differential Diagnosis. A similar condition, lichen spinulosus (135), shows a very similar histologic picture except that the keratin plug may protrude more substantially above the skin surface and contain one or more hair shafts. Lesions similar to keratosis pilaris may also be seen in patients treated with vemurafenib (136), as well as phrynoderma (vitamin A deficiency; see Chapter 16), though the follicular keratin plug is said to be parakeratotic.
Principles of Management. Keratosis pilaris is primarily managed by topical keratolytics containing ammonium lactate, salicylic acid, or retinoids. Severe cases with clinical erythema may necessitate topical corticosteroids.
Trichostasis Spinulosa
Clinical Summary. Trichostasis spinulosa is a fairly common condition that may present clinically as raised follicular spicules or as open comedones or be inapparent (137). It occurs predominantly on the face, nose, or cheeks of middle-aged and older individuals, but it has been reported in a pediatric patient (137). It may be pruritic. Generalized trichostasis spinulosa has been reported in a patient with chronic renal failure (138).
Histopathology. Affected hair follicles demonstrate retention of small hair shafts within a dilated infundibulum, sometimes enveloped in a keratinous sheath (Fig. 18-17). As many as 20 or more hair shafts may be trapped in this way, often projecting above the skin surface. A perifollicular mononuclear infiltrate may be present. In the clinical setting, the follicular plugs may be easily extracted with fine forceps or a comedone extractor and examined microscopically, demonstrating a cluster of vellus hairs within a keratinous plug.

Figure 18-17 Trichostasis spinulosa. A dilated follicular infundibulum with retention of multiple vellus hair shafts. The granular blue material represents resident bacteria of the follicle.
Pathogenesis. The etiology of trichostasis spinulosa is not known for certain. The retained hairs are normal telogen hairs, which suggests a normally functioning follicle. Congenital dysplasia of the hair follicles and external factors such as dust, oils, ultraviolet light, heat, and irritants have been proposed (137). One hypothesis is that hair shaft entrapment is the result of hyperkeratosis in the follicular infundibulum, thus producing an obstruction to normal hair shedding.
Differential Diagnosis. Trichostasis spinulosa may clinically resemble cutaneous spicules seen in multiple myeloma, but in the latter condition the follicles are filled with amorphous eosinophilic material, representing precipitated serum monoclonal protein, rather than hair shafts (139).
Principles of Management. Mechanical removal of entrapped hair shafts may be achieved with forceps, a comedone extractor, waxing, or adhesive strips marketed for open comedones. Topical keratolytics or retinoids may also be beneficial.
DISEASES OF HAIR FOLLICLES LEADING TO HAIR LOSS: ALOPECIA
A discussion of alopecia generally pertains to loss of hair on the scalp, although other body sites may be affected, as can be seen in AA, trichotillomania, telogen effluvium, and lichen planopilaris (LPP), to name a few. A useful way to broadly classify the alopecias is to divide them into nonscarring and scarring types depending on their (relative) propensity to culminate in permanent hair loss. An algorithmic approach to the alopecias was presented in an excellent paper by Solomon (140). Sometimes classification of an individual case of alopecia can be complicated by the coexistence of more than one process. For example, scarring alopecia can occur in a patient with underlying androgenetic alopecia (AGA).
Some types of alopecia are discussed elsewhere in this book, such as lupus erythematosus (Chapter 10), syphilitic alopecia (Chapter 22), perifolliculitis capitis abscedens et suffodiens (Chapter 21), folliculitis (acne) keloidalis nuchae (Chapter 21), and alopecia neoplastica (Chapter 36). Although hair shaft disorders result in alopecia, a diagnosis is often made on clinical grounds and on the basis of examination of hair shafts rather than biopsy material; therefore, a discussion of hair shaft disorders is not included here but can be found in one of several review articles (141–143). Alopecia that is easily diagnosed in the clinical setting on the basis of history, physical examination, and microscopic assessment of gently pulled hairs, thereby negating the need for a scalp biopsy, is also not discussed; these include postoperative pressure-induced alopecia, temporal triangular alopecia, and loose anagen syndrome.
General Considerations in the Histologic Evaluation of Alopecia
The Scalp Biopsy
A punch biopsy or incisional biopsy extending into fat to include terminal hair bulbs is necessary for proper evaluation of alopecia. A punch biopsy is easier for the clinician to perform, and most scalp biopsies are submitted as such. It is generally accepted that at least a 4-mm punch be used to obtain a sufficient number of hairs for study. In addition, many of the published quantitative parameters for diagnosing different forms of alopecia are based on a 4-mm punch; so this is the one that is generally recommended (144).
Controversy still exists regarding the manner in which the punch biopsy should be sectioned: vertically, where the cylinder of tissue is bisected longitudinally, which is the standard way of sectioning punched specimens procured for other cutaneous disorders, or horizontally (aka transversely), in which the skin cylinder is “bread-loafed,” which is the method pioneered by Headington (145). Horizontal sectioning enables visualization of all the follicles in the specimen (Fig. 18-18), whereas it has been estimated that conventional vertical sections demonstrate only 10% to 15% of the follicles in the sample (145). Dermatopathologists appear to be polarized with regard to which method of sectioning is preferable. Of dermatopathologists who favor vertical sections, some feel that they can glean as much information if serial sections are obtained, and some feel uncomfortable interpreting hair follicle anatomy and pathology in a horizontal orientation. A notable disadvantage of transverse sections is the inability to properly evaluate the epidermis (140). However, when a transversely sectioned biopsy is cut through, a portion of the epidermis can usually be seen and evaluated, albeit tangentially.
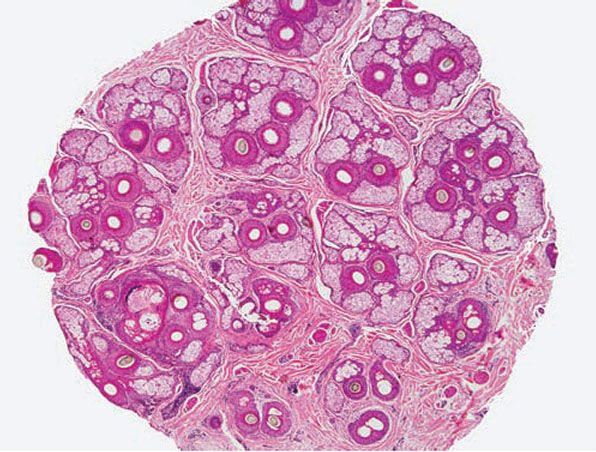
Figure 18-18 Horizontal section, scalp. This 4-mm punch biopsy was sectioned horizontally (aka transversely), enabling visualization of every follicle in the specimen. At this level, through the mid-dermis near the isthmus, arrangement of pilosebaceous structures into “follicular units” is apparent.
Some authors have recommended that two 4-mm punch biopsies be performed from the area of disease activity, and that one biopsy be sectioned transversely and the other sectioned vertically. The latter is bisected at the time of the biopsy, with one half sent for routine histology (embedded with the two halves of the transversely sectioned specimen), and the other half processed for direct immunofluorescence (146). In some forms of nonscarring alopecia such as telogen effluvium or early AGA, in which the histologic findings may be subtle, it may be helpful to take one specimen from the involved vertex of the scalp and one specimen from the occiput; upon comparing the two, one should find the occipital scalp to be relatively normal in AGA and equally involved in telogen effluvium. Certainly a single biopsy is preferable from a patient morbidity standpoint, and this may be sufficient (147).
For diagnosing cicatricial alopecia, the recommendation is for one 4-mm punch biopsy specimen from the edge of an involved area where there is new hair loss, a positive hair pull test, or inflammation as evidenced by clinically apparent erythema (148). If the goal of the biopsy is to assess the potential for hair regrowth (prognosis), then the optimal area to sample is an older, more central area with a “burnt-out” appearance. In 2011, the HoVert technique was introduced as a way to obtain horizontal and vertical sections from a single punch biopsy, and was touted to aid in the diagnosis of scarring alopecias, particularly LPP and discoid lupus erythematosus (149). In this technique, a 4-mm punch biopsy is transected 1 mm below the epidermis to create an epidermal disk that is bisected, allowing visualization of the epidermis in vertical orientation. Transverse sections are cut from the remainder of the specimen. The “Tyler technique” also allows for horizontal and vertical sections to be prepared from a single punch biopsy; the specimen is first bisected vertically, and then one half is sectioned horizontally (150).
In the past, serial horizontal sections would result in numerous sections and multiple slides to review, typically 12 to 20 (147). A proposed method for improving the convenience of transverse processing recommended trisecting or quadrisecting the specimen, inking one side of each resultant disk of tissue in a consistent orientation, and then embedding them all in a single cassette so that several microanatomic levels can be viewed on a single slide (147). My personal experience with this technique is that, although convenient, it sometimes results in loss of tissue (often critical tissue) because laboratory personnel significantly efface some of the tissue pieces to obtain a “good” slice of all of the tissue disks. This can occur particularly when the pieces of tissue are embedded at slightly different depths in the paraffin block.
I have found that a safer way to process the punch specimen to avoid loss of tissue is to bisect it transversely approximately 1 mm below the epidermal surface, then ink the cut surfaces, and embed the two pieces together so that the inked surfaces are cut first, a method previously advocated (140,145). Deeper levels from the block reveal sections that approach the epidermis for one piece of tissue and the subcutis for the other piece of tissue.
The ideal way to procure a punch biopsy for transverse processing is to orient the punch tool parallel to the direction of hair growth, decreasing the frequency that hairs are transected by the biopsy procedure. The result is a cylinder of scalp skin in which the sides of the cylinder are not exactly perpendicular to the epidermal surface. With this in mind, when the specimen is bisected 1 mm below the epidermis, the cut should be angled parallel to the epidermal surface (not perpendicular to the sides of the tissue cylinder) so that each histologic section will show all of the follicles at roughly the same microanatomic level, which facilitates their interpretation. As a consequence, however, follicles and hair shafts will appear ovoid in shape under the microscope rather than round.
My personal preference is to interpret transverse sections whenever possible, and this chapter emphasizes this method. Transversely sectioned scalp biopsies offer the following advantages (140): (a) all of the follicles in the biopsy specimen can be seen and studied, (b) it allows for rapid evaluation of hair density and follicular units, (c) it allows for accurate assessment of follicle size, (d) pathologic alterations at different levels of the follicle can easily be evaluated, and (e) it facilitates quantitative interpretation of the biopsy. In my experience, it is also a more sensitive technique for detecting tinea capitis when only scattered follicles are involved. Horizontal sections are ideal for evaluating the number of viable follicles and estimating prognosis for hair regrowth. As previously mentioned, interpretation of horizontal sections requires a thorough understanding of hair follicle microanatomy, as well as the normal hair cycle. These are superbly described in Chapter 3 and in other sources (140,145,151,152) and are not reiterated here.
Recently introduced terms include exogen—a distinct phase of the hair cycle in which the hair shaft is actively shed, most likely by a proteolytic mechanism (153)—and kenogen—referring to the empty hair follicle after the telogen hair is shed (154). Kenogen is believed to represent a physiologic rest period for the follicle prior to initiation of a new growth phase.
Terminology and Definitions
Solomon and Templeton (155) noted that “working definitions” are “important in the microscopic evaluation of alopecia,” although “these definitions are arbitrary and therefore debatable”. Although authors differ in the definitions they use to characterize hair follicles, the following definitions are commonly employed. A terminal hair was designated by Headington (145) as having a hair shaft diameter of 0.06 mm or greater and a vellus hair as having a hair shaft diameter of 0.03 mm or less. Between terminal and vellus hairs are intermediate hairs (aka indeterminate), whose shaft diameter, i, is defined as 0.06 > i > 0.03 mm (Fig. 18-19). A micrometer is sometimes needed to accurately assess hair shaft diameter, although without actually measuring them, hair follicles can be classified by comparing the shaft size to the width of the inner root sheath; the diameter of a vellus hair shaft should be less than or equal to the thickness of the inner root sheath (Fig. 18-19). Qualitatively, terminal hairs have their bulbs anchored in the subcutaneous fat, whereas vellus hair bulbs are in the mid- to upper dermis.
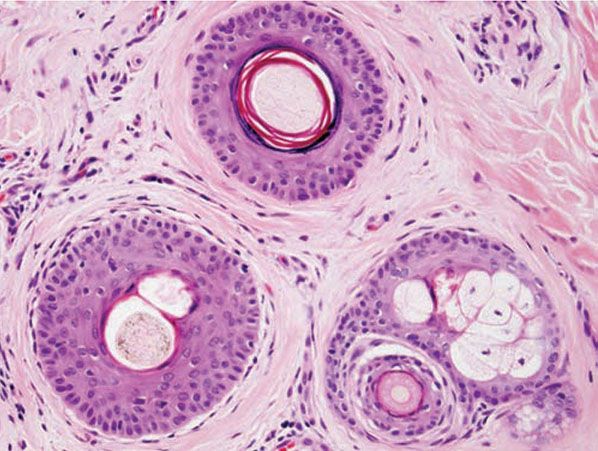
Figure 18-19 Normal variation in hair diameter (horizontal). This level is through the infundibulum of the terminal follicle, defined by a hair shaft diameter that is 0.06 mm or greater (top). The vellus hair (lower right) is readily identifiable by a shaft diameter that is equal to or less than the thickness of the inner root sheath (≤0.03 mm). An intermediate hair (lower left) has a shaft diameter that is between the other two.
True vellus hairs lack melanin pigment and are small throughout their existence, whereas miniaturized vellus hairs are terminal hairs that have decreased in size. Because these are histologically identical, it is not necessary to distinguish them for the purpose of diagnosing alopecias; henceforth in this chapter, the term vellus hairs will encompass true vellus hairs and miniaturized terminal hairs. Although some authors group intermediate hairs with the terminal hair population for the purpose of quantification, I prefer to include them with the vellus group because more often they represent a transitional follicle undergoing the process of miniaturization to a vellus hair. Reversal of the process via therapy with minoxidil or finasteride can confuse the picture, however. A cross section at the lower infundibular level is optimal for visualizing vellus hairs, allowing one to accurately count terminal and vellus hair shafts to determine the terminal-to-vellus hair ratio.
In determining the telogen count or anagen-to-telogen ratio, the term telogen actually encompasses all phases of the nonanagen follicle, including telogen, catagen, and telogen germinal unit (Fig. 18-20), because they all represent stages in a continuum: the irreversible process of hair follicle regression and shedding. For instance, a catagen follicle, with an epithelial column in the subcutis and/or lower dermis, often shows a telogen hair near the isthmus. Although vellus hairs also cycle, only terminal-sized follicles in catagen and telogen are counted as part of the regressing/resting hair population. The telogen count derived histologically, using these definitions, reflects the count obtained in the clinical setting by forcible hair pluck (using a rubber-tipped hemostat) because this method is unsuccessful at dislodging the smaller vellus hairs (151,156).
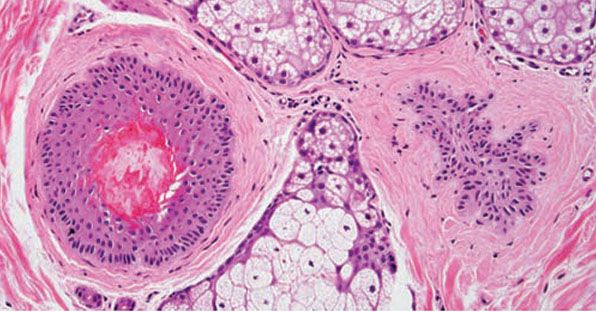
Figure 18-20 Normal resting follicles (horizontal). The follicle at left is a telogen follicle, identifiable by brightly eosinophilic trichilemmal keratinization of the proximal hair shaft. This structure is the histologic correlate of easily displaced telogen hairs viewed by microscopy in the clinical setting, the so-called club hairs. To the right is a telogen germinal unit, which represents the secondary hair germ. This structure is found in the mid- to upper dermis and is characterized by follicular epithelium with radial projections. In vertical sections, it is sometimes visible just beneath the telogen hair.
A cross section through the isthmus is optimal for assessing the number of telogen and catagen hairs and telogen germinal units. This telogen count should be interpreted in the context of a second count at the infundibular level to assess terminal and vellus hairs because follicles in telogen or catagen according to their appearance near the isthmus show a shaft in the infundibulum that looks identical to that of a terminal anagen follicle; that is, a hair shaft with a diameter of 0.06 mm or greater situated in the infundibular hair canal. Often the infundibular portion of the hair shaft, untethered by the inner root sheath, is missing, presumably carried away by the microtome blade. In this situation, the pathologist must rely on the caliber of the follicular outer root sheath and the diameter of the empty hair canal to extrapolate the size of the hair shaft and obtain more accurate counts.
Miniaturization and conversion to catagen and telogen leave many collapsed fibrous root sheaths in the subcutis (Fig. 18-21). Also known as “stelae” and “fibrous streamers,” they are found in conditions in which there is follicular miniaturization (i.e., AGA) or conditions with increased numbers of catagen and telogen hairs, such as telogen effluvium and trichotillomania.

Figure 18-21 Fibrous streamer (horizontal). Also known as stelae, these collapsed fibrous sheaths are subcutaneous evidence of hairs that have moved upward through the process of miniaturization or conversion to telogen. They consist of concentrically arranged bands of collagen with numerous capillaries and an increased number of mast cells. (From Elder DE, Elenitsas R, Rubin AI, et al. Atlas and synopsis of Lever’s histopathology of the skin, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2013, with permission.)
Normal Parameters
Normal parameters as determined by examination of transversely sectioned scalp biopsies have been published in various sources and are typically based on a 4-mm punch biopsy. Most were from studies of white subjects, but ethnic differences in “normal” values have been demonstrated, and this must be taken into consideration when evaluating a scalp biopsy. Whites without alopecia should have approximately 40 total hairs in a 4-mm punch biopsy, with roughly 20 to 35 terminal hairs and 5 to 10 vellus hairs (144,145,156,157). The normal terminal-to-vellus hair ratio for adult scalp is 3:1 to 4:1 (145) but at least 2:1 (158). Another study found a normal value of 7:1 (157).
Hair density in African Americans was found to be significantly lower than in whites, with an average of 18 terminal and 3 vellus hairs in a 4-mm punch biopsy (159). However, the follicles are typically larger (160). In Koreans, the follicular density was found to be significantly lower than in whites and blacks (161).
Hair follicles on the scalp are arranged in follicular units—bundles of hairs naturally found together. They form hexagonal modules separated from one another by intervening collagen and are best visualized by transverse sections through the upper dermis near the isthmus (Fig. 18-18) (162). There are typically 10 to 12 follicular units in a 4-mm punch (140). Each follicular unit normally contains two to five terminal hairs and zero to two vellus hairs, along with sebaceous glands and arrector pili muscle (144).
Nonscarring Alopecias
Alopecia that is nonscarring clinically shows intact follicular ostia and histologically shows a normal density of hair follicles (163,164). Despite being categorized as “nonscarring,” conditions such as AGA, long-standing AA, and traction alopecia can lead to irreversible loss of hair follicles. Transverse sections are particularly useful in the histologic diagnosis of nonscarring alopecias because the anagen-to-telogen ratio and terminal-to-vellus hair ratio may be crucial in making an accurate diagnosis. In particular, female patients with a fairly diffuse, nonscarring alopecia can be a diagnostic dilemma based on clinical grounds alone, and a biopsy can help. The main diagnostic possibilities in this setting are (a) female-pattern hair loss, (b) acute and chronic telogen effluvium, (c) diffuse AA, and (d) loose anagen syndrome (165,166). The salient features of the most common nonscarring alopecias are listed in Table 18-1.
Alopecia Areata
Clinical Summary. AA is clinically characterized by complete or nearly complete absence of hair in one or more circumscribed areas of the scalp (Fig. 18-22) (167,168). Clinical inflammation, typically manifested by erythema, is not obvious, and the follicular openings are preserved, a clinical finding that allows the examiner to make the assessment of a nonscarring alopecia. In active areas of involvement, hair shedding is seen, as well as some short, fractured hair shafts, including the pathognomonic “exclamation point” hair. Complete scalp involvement (alopecia totalis) may occur suddenly or through prolonged, progressive disease. Complete or nearly complete loss of all body hair (alopecia universalis) can also occur. Involvement of the eyebrows and eyelashes and regularly spaced pits on the surface of the nails may be seen. The majority of patients with localized disease undergo spontaneous resolution, but others show persistent disease, and a few patients have permanent hair loss. “The only predictable thing about the progress of alopecia areata is that it is unpredictable” (169), which means essentially that one cannot reliably predict which patients will have limited disease with spontaneous resolution and which will have recurrent disease or chronic, severe disease.

Figure 18-22 Alopecia areata. Patchy alopecia characteristic of alopecia areata, ophiasis pattern. Despite the microscopic lymphocytic infiltrate that is sometimes present, the areas lack clinical evidence of inflammation, namely erythema.
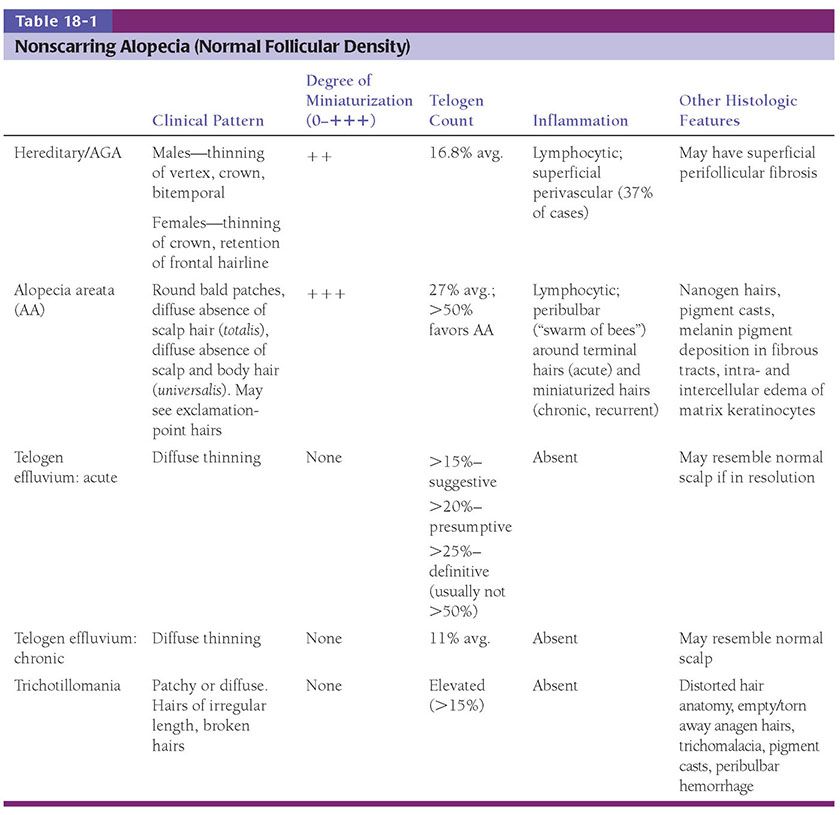
Histopathology. The diagnostic pathologic feature is peribulbar lymphocytic inflammation (“swarm of bees”) affecting anagen follicles (Fig. 18-23) or follicles in early catagen (Fig. 18-24). The inflammatory assault on anagen follicles induces a premature conversion to catagen. Consequently, the number of catagen and telogen follicles found may be marked, approaching 100% (Fig. 18-25) (170). Follicles may enter a persistent phase of telogen in which the hair shaft has already been shed, manifested by the telogen germinal unit (170). As follicles enter catagen, the lymphocytic infiltrate may persist around the epithelial remnant of the receding follicle and also within and surrounding the fibrous tracts (Fig. 18-24). Telogen hairs show little to no perifollicular inflammation.
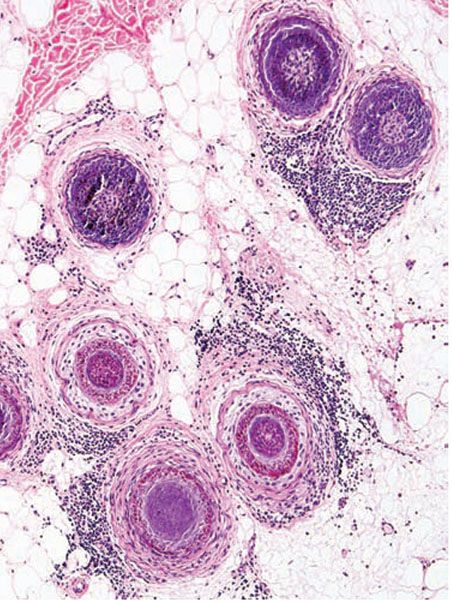
Figure 18-23 Alopecia areata (horizontal). Classic peribulbar lymphocytic inflammation likened to a “swarm of bees.”
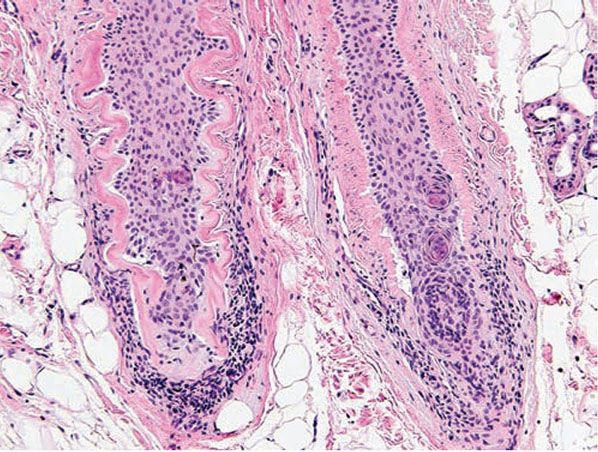
Figure 18-24 Alopecia areata (vertical). Lymphocytic inflammation surrounding catagen follicles, which are distinctive because of their eosinophilic “glassy” membrane and lack of a hair shaft.

Figure 18-25 Alopecia areata (horizontal). In subacute alopecia areata, terminal anagen hairs attacked by inflammation will undergo conversion to telogen. A biopsy at this stage may show almost 100% terminal telogen hairs.
Lymphocytes may also be seen sparsely infiltrating the matrix epithelium of anagen follicles, inducing damage to the matrical cells that includes intra- and intercellular edema, cellular necrosis, and microvesicle formation. One of the earliest findings was shown to be a loss of structural integrity of bulbar keratinocytes in the central part of the supramatrical bulb and shrinkage of hair bulbs toward a club shape (171). As a result of injury to bulbar melanocytes and keratinocytes, pigment casts, which are clumps of melanin pigment, may be found within the dermal papilla, the sheath of miniaturizing or regressing follicles, the follicular epithelium, or fibrous tracts (158,172). Pigment casts are more often found in trichotillomania; one interpretation of their presence in the context of AA is that here too they represent external manipulation of the hair (35). Pigment casts have also been found in postoperative pressure-induced alopecia, leading those authors to postulate that they resulted from the sudden conversion of follicles from anagen to catagen, as occurs in trichotillomania and AA (173).
The inflammatory assault may produce dysmorphic follicles and shafts. Anagen hairs not sufficiently damaged so as to enter prematurely into catagen may continue to manufacture a hair shaft, one that may be small, distorted, and often nonpigmented (termed trichomalacia). The hair shaft may taper to a point so tiny and fragile that it fractures. Small, abnormal follicles called nanogen hairs are a distinctive finding in long-standing cases (Fig. 18-26) (160). They are difficult to categorize as anagen, catagen, or telogen hairs, and in transverse sections they show only a minute, incompletely keratinized hair shaft or no shaft at all.
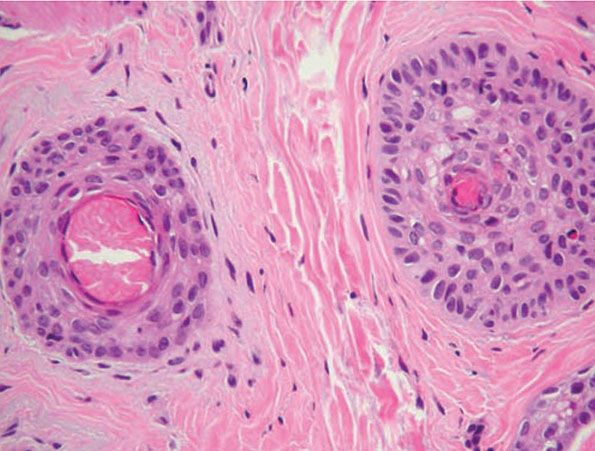
Figure 18-26 Alopecia areata (horizontal). Dystrophic hairs. The follicle at left has no shaft at all; the inner root sheath completely fills the canal. The follicle to the right is producing only a minute, “pencil-point” hair shaft that will break easily.
As telogen follicles reenter anagen, they again come under attack from pathogenic lymphocytes, which precipitate premature conversion to catagen once again, so that anagen duration becomes shorter and shorter and the follicles begin to miniaturize (Fig. 18-27). As the follicles decrease in size, they become situated more superficially, although often deeper than normal vellus hairs, with their bulbs situated in the mid- to lower dermis (158). With disease chronicity, most of the hairs become miniaturized. Miniaturization and conversion to catagen and telogen leave many collapsed fibrous root sheaths in the subcutis.
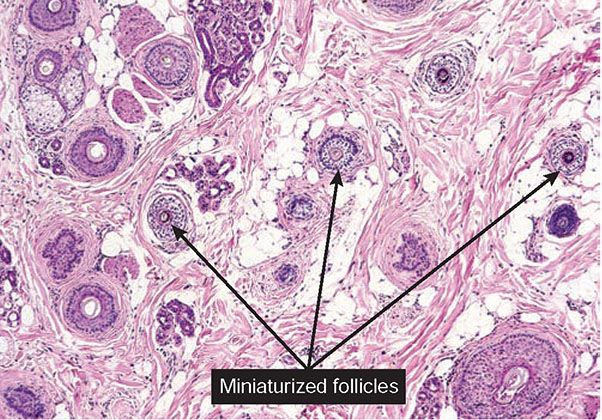
Figure 18-27 Alopecia areata (horizontal). In long-standing alopecia areata, there is an increased number of catagen and telogen follicles, and follicular miniaturization begins to occur. The combination of increased resting and miniaturized hairs is characteristic of alopecia areata, even when peribulbar inflammation is not present. (From Elder DE, Elenitsas R, Rubin AI, et al. Atlas and synopsis of Lever’s histopathology of the skin, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2013, with permission.)
Whiting found that transversely sectioned scalp biopsies showed the diagnostic features of AA more often than vertically sectioned biopsies (174). He also published mean quantitative hair counts for horizontally sectioned scalp biopsies of AA. The mean terminal-to-vellus hair ratio was similar to that seen in AGA (1.1:1), reflecting extensive miniaturization. The mean anagen-to-telogen ratio was 73%:27%, and the total hair count (mean of 27 hairs) was 33% less than in the control group, with more severely affected patients (alopecia universalis) at the lower end of the spectrum. Others have also found that the follicular density can decrease in severe alopecia totalis and universalis of long duration (a decade or more), with scars replacing some of the follicular sheaths (175).
Whiting and others have emphasized the use of follicular counts to aid in the diagnosis of AA when the characteristic peribulbar inflammation is missing, with a high percentage of catagen or telogen hairs and miniaturized hairs as a strong sign of AA (Fig. 18-24) (172,176). In one study, the presence of eosinophils around the bulb and within fibrous tracts was found to be a helpful diagnostic feature of AA, identifying it in 38 of 71 patients. Eosinophils were found in fibrous tracts 44% to 50% of the time (172,177). Some plasma cells may also be present. Dilated follicular infundibula filled with orthokeratin, resembling “follicular Swiss cheese” on horizontal sections, is another histologic clue to the diagnosis of AA and correlates with yellow dots seen with dermoscopy (178,179).
Some authors claim that in long-standing AA, the inflammatory infiltrates appear to diminish (180), whereas others believe that the degree of inflammation found on biopsy does not depend on disease duration, citing cases of long-standing AA that showed a great deal of inflammation (158). In a reappraisal of the histopathology of AA, Whiting suggested that the key factor in the histologic picture was the duration of the attack, with lymphocytes surrounding mainly terminal hair bulbs in acute episodes and involving mainly miniaturized hair bulbs in chronic, recurrent disease (176).
Pathogenesis. The exact pathogenesis of AA is not known, but substantial evidence exists to support a role for genetic factors, nonspecific immune- and organ-specific autoimmune reactions, and environmental triggers (169,181,182). The antigenic stimulus for autoimmune attack may be the follicular keratinocyte, melanocyte, or dermal papilla. Advances in the understanding of AA in the last decade have benefited from animal models of the disease (183), including the C3H/HeJ mouse (184), the Dundee experimental bald rat (185), and the Smyth chicken (186).
Familial cases of AA are well recognized, suggesting a genetic predisposition (187). A family history of AA reportedly ranges between 10% and 42% (188), with higher incidences in patients with disease onset early in life and in identical twins (189). AA has been associated with both HLA class I and class II antigens, with different forms and severity of AA found to be associated with certain HLA types (169,190). AA has been associated with many conditions, including Down syndrome, atopy, vitiligo, thyroid disease, pernicious anemia, diabetes, myasthenia gravis, lupus erythematosus, rheumatoid arthritis, ulcerative colitis, lichen planus, polymyalgia rheumatica, Candida endocrinopathy syndrome, and idiopathic thrombocytopenia purpura (169,191).
In a study using indirect immunofluorescence, autoantibodies to various parts of the anagen hair follicle were found in AA patients (192). Most commonly targeted were the outer root sheath, then the matrix, inner root sheath, and hair shaft. Tobin et al. (193) also found serum autoantibodies to pigmented hair follicles in 100% of AA patients but in only 44% of control patients.
Earlier experiments showed the peribulbar infiltrate to be composed predominantly of CD4+ cells (helper T lymphocytes) (194). Subsequently, CD8+ cells (suppressor-cytotoxic T lymphocytes) were implicated in the pathogenesis of AA, with experiments supporting a cooperative role between CD8+ and CD4+ T cells (195). Expression of cell adhesion molecules in the matrix epithelium, dermal papilla, and adjacent vessels has been demonstrated, suggesting a mechanism for leukocyte binding (196).
The immune system of the hair follicle is unique and differs from that of the surrounding skin (197). The epithelial portion of the proximal anagen hair is immune privileged; the inner root sheath and hair matrix do not express major histocompatibility complex (MHC) class I antigens (197,198). A theory put forth by Paus, an “immune privilege collapse model,” suggests that in AA, the body’s immune system may begin to recognize immune-privileged hair follicle antigens as a result of upregulated MHC molecules or downregulation of local immunosuppressive factors (197,198). Also, a role for the peripheral nervous system has been postulated in AA, via neuropeptides with pro- and anti-inflammatory properties released in the vicinity of critical areas of the hair follicle (199).
Differential Diagnosis. Alopecia syphilitica may closely mimic active AA (200). Features that help to distinguish syphilitic alopecia include lymphocytes situated near the isthmus, the presence of plasma cells, an endothelial reaction, interface dermatitis, and neutrophils in the stratum corneum (177,200). AA may also look identical to the patchy, nonscarring alopecia that occurs in the setting of systemic lupus erythematosus, which shows mononuclear cell inflammation around terminal and miniaturized anagen hair bulbs, as well as an increased percentage of resting hairs, sometimes approaching 100% (160). Features favoring lupus erythematosus include increased dermal mucin, focal basal vacuolization of infundibular epithelium, and inflammation around blood vessels and eccrine glands, particularly when the infiltrate is dense. Although AGA shows diminution of follicular size, dermal infiltrates that are present in this condition are usually superficial, perivascular, and peri-infundibular. Using a comparison of clinical and histologic features, one paper discussed problematic differential diagnoses with respect to AA (201).
Principles of Management. Alopecic patches may resolve spontaneously. For refractory patches, intralesional corticosteroids are a very effective treatment. For more widespread involvement or for patients averse to needles, topical high-potency steroids are a reasonable alternative, though penetration to the level of the hair bulbs is an issue. A topical retinoid may be added to the regimen to facilitate deeper penetration of the steroid. When larger areas are involved (i.e., alopecia totalis), topical immunotherapy with squaric acid dibutylester or dinitrochlorobenzene (DNCB) may be a better option.
Trichotillomania
Clinical Summary. Trichotillomania is a condition in which patients pull or manipulate hair from the scalp or other body sites (202). For example, persistent rubbing of an area of the scalp that may be pruritic or compulsive avulsion of hair shafts can lead to zones of alopecia characterized by sparse, ragged, broken stubble. Hair shaft breakage and loss may be associated with damage to the scalp, as evidenced by erosions or crusts. Trichotillomania occurs most often in children and adolescent girls. In children under the age of 6 years, the disorder may be more benign and self-limited (203). In teens and adults, trichotillomania is more likely to be associated with psychopathology. It has features in common with obsessive-compulsive disorder (204), and in one study a large proportion of patients with trichotillomania also had comorbid self-injurious habits (205). Trichotillomania is part of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), classification of psychiatric disorders as a recurrent failure to resist the impulse to pull hair, with rising tension followed by relief or gratification after pulling out hair (205).
Histopathology. In horizontally sectioned biopsies of trichotillomania uncomplicated by the coexistence of other types of alopecia, the density of hair follicles is normal, as is the terminal-to-vellus hair ratio. The diagnostic finding, when seen, is distorted hair follicle anatomy, without inflammation (Fig. 18-28) (158). Specifically, the pulling of hairs can leave behind empty anagen follicles and “torn-away” follicles, the result of plucked hair shafts that retain parts of the hair matrix and root sheaths (Fig. 18-29). Additional microscopic evidence of traumatic injury was published by Royer and Sperling (206) as the “hamburger sign,” describing a vertically oriented split in the hair shaft containing proteinaceous material and erythrocytes, resembling a hamburger within a bun (Fig. 18-30). Distorted hair follicle anatomy may assume the appearance of other food items (e.g., hot dog) (207). Damaged follicles enter the resting phase, leading to an increase in the percentage of catagen and telogen hairs, as high as 75% (Fig. 18-31) (208). Often the hairs do not become normal catagen hairs and appear distorted and abnormal (158).

Figure 18-28 Trichotillomania (vertical). The hair canal is distorted in a spiral configuration, most likely secondary to twisting of the hair. Pigment casts, derived from bulbar or hair shaft melanin, are present. (From Elder DE, Elenitsas R, Rubin AI, et al. Atlas and synopsis of Lever’s histopathology of the skin, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2013, with permission.)
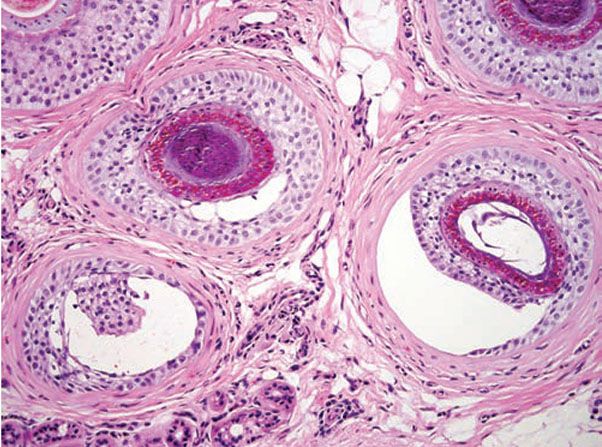
Figure 18-29 Trichotillomania (horizontal). The follicle at left shows a rim of outer root sheath epithelium that remains after the hair was forcefully pulled, taking with it the inner root sheath and part of the outer sheath. At right, the follicle is partially avulsed and the shaft that is normally tightly anchored to the inner root sheath at this level is gone.
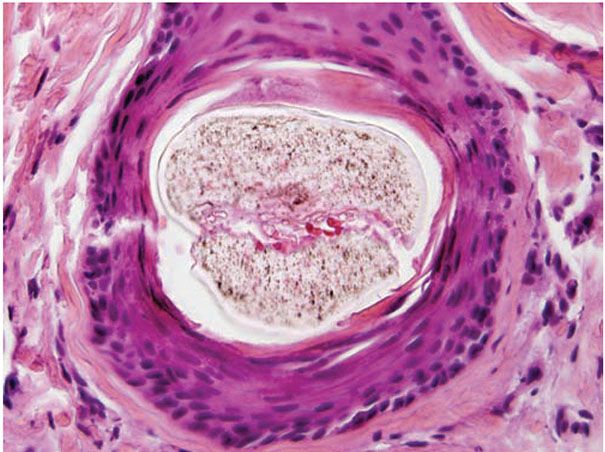
Figure 18-30 Trichotillomania (horizontal). A rare but diagnostic finding in trichotillomania is longitudinal splitting of the hair shaft. When blood and proteinaceous debris are seen in the split, it is known as the “hamburger sign.”
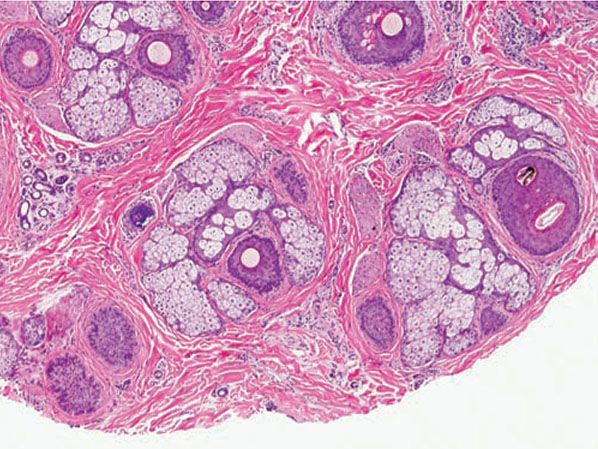
Figure 18-31 Trichotillomania (horizontal). Low-power view reveals an increased number of “resting” follicles, with catagen and telogen hairs, and telogen germinal units. A follicle to the far right contains a black pigment cast.
Pigment casts, which are clumps of melanin pigment, may be seen in the hair papilla and peribulbar connective tissue. They are also commonly seen in the upper portion of the hair follicle (Fig. 18-28) as a result of pigmented matrical cells being deposited distally as the hair is plucked. Pigment casts are due to injury to the hair matrix, although some authors have theorized that they result from the sudden conversion of anagen to catagen (173). Hair shaft changes, termed trichomalacia, may be seen. Characterized by diminished size, distorted and odd shape, and irregular pigmentation of the shaft, trichomalacia is additional evidence of trauma to the matrix (158,208
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree