Fig. 16.1
(a–c) Preoperative 19-year-old patient with contour deformity left axilla and upper arm after car accident
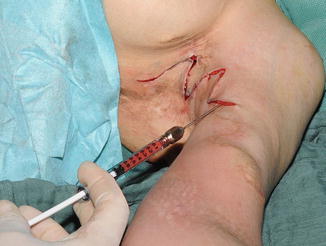
Fig. 16.2
Scar correction surgery was performed by local advancement flaps after tissue expansion, z-plasties, and extensive lipofilling
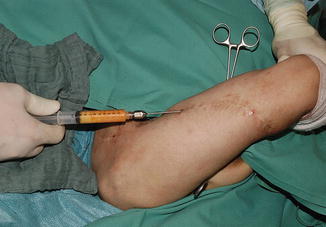
Fig. 16.3
Lipofilling of the upper arm scars
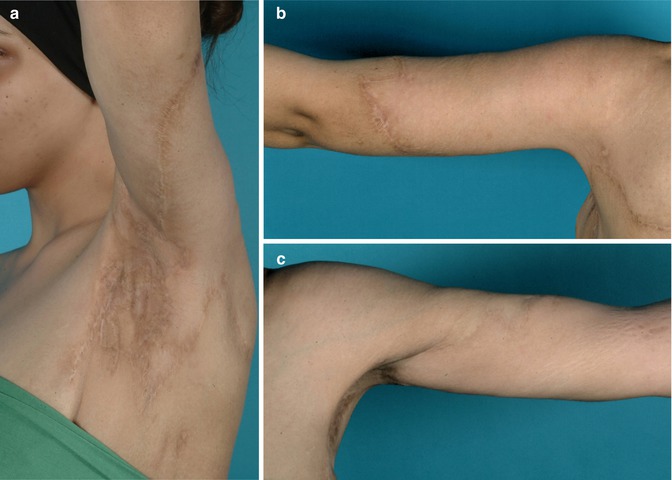
Fig. 16.4
(a–c) Postoperative results
16.3 Risks of Fat Grafts
The lipofilling procedure itself is a rather straightforward procedure with manageable complication rates provided that the performing specialist is well trained, follows general surgical rules, and respects anatomic landmarks [13, 14]. The incidence of complications notably increases with the volume of liposuction; therefore, the specialist should rather perform a staged procedure than a single high volume lipotransfer [15]. As in any other surgery, minor complications include infections, edemas, ecchymosis, dyschromias, or seromas at both the site of harvest and site of injection. Serious and in certain circumstances life-threatening complications are intoxication from local anesthetics, sepsis, necrotizing fasciitis, perforation of organs, hyper- or hypohydration, multisystem failure due to liposuction of exorbitant volumes, and thromboembolisms [16]. For the liposuction procedure one death in every 5,000 operation was reported [17, 18]. A feared complication is embolism, which is believed to result from mobilized fat penetrating damaged blood vessels mostly clotting lung vessels [19]. A study on Wistar rats showed that the risk of fat embolism after liposuction is higher compared to the control group and even higher in rats which underwent liposuction and subsequent lipofilling [20].
Apart from the abovementioned complications, critics rather focused on other aspects of fat grafts including fat necrosis and resorption, radiological interference, and the currently intensely debated carcinogenicity.
16.4 Fat Necrosis and Graft Resorption
Fat necrosis after grafting was complained already in 1919 when Marchand conducted histological examinations of fat transplants and described fat absorption and necrosis in this newly invented method, contrary to early optimism [21]. In 1923, Neuhof stated that transplanted fat would undergo necrosis and eventually be replaced by new fat or fibrous tissue [22, 23]. Today the reported survival rate of fat grafts range from 40 to 80 % [24]. Results depend on the performing surgeon, technique, and many more factors. While fat necrosis and resorption represent no severe risk to the patient, they still are an often mentioned issue resulting in serial lipofilling procedures, decreasing patient’s satisfaction, and thereby fuel the critics stating that fat is an inadequate filling material. New fat processing methods, preoperative or simultaneous injection of growth factors, and other techniques promise to increase fat graft vitality also in the long term [22]. Supporters of fat grafting claim that fat grafts maintain volume by cell recruitment to the recipient site or the differentiation and proliferation of mesenchymal stem cells. The simplest way to counteract graft resorption is to transplant more fat than actually required. Eighty-seven percent of the plastic surgeons participating in a survey tend to overcorrect contour deformities to address an expected fat resorption with 31 % of the patients requiring a reinjection and the satisfaction of patients declining with time [6]. To avoid fat necrosis, experts suggest small volume injections in thin layers to grant sufficient perfusion at the recipient site [25, 26].
16.5 Interference with Radiological Cancer Diagnosis
Fat necrosis not only leads to the loss of volume and possible contour irregularities but was also discussed as a possible disturbing factor in the radiological detection of breast cancer. In 1987, the American Society of Plastic and Reconstructive Surgeons Ad-Hoc Committee on New Procedures [27] deplored the augmentation of breasts by autologous fat with the major concern being fat necrosis and calcifications which would obscure the detection of breast cancer by mammography. In the following years diverse studies tried to elucidate the actual potential of fat grafts to hamper early breast cancer diagnosis. The largest study by Delay et al. published in 2009 [13] came to the conclusion that oily cysts, calcifications, or complex cysts as a result of fat necrosis are well distinguishable from malign lumps. Pierrefeu-Lagrange et al. [28] also stated that lipofilling does not affect the radiological follow-up of patients with breast cancer history. In agreement with the aforementioned publication, Constantini et al. postulated that fat grafts do not interfere with early cancer diagnosis. Corresponding to the experience of Constantini et al. [29], ultrasound helps to detect oil cysts, whereas fat necrosis identification by magnet resonance imaging (MRI) is more reliable. Many more articles concentrate on the radiological interference of fat grafts with most of the authors concluding that fat grafting does not cause difficulties in discriminating benign lesions induced by lipofilling from malign tumors [30]. According to the clinical data, the American Society of Plastic Surgeons Fat Grafting Task Force [31] revitalized the statement earlier made in 2009 by stating that fat grafts are a considerable therapy option for breast augmentation, which can potentially interfere with breast cancer detection, although hard evidences for this suspicion could not be found until then.
16.6 Carcinogenic Risk
Aside from a possible radiological misdiagnosis of breast malignancies, the carcinogenic potential of fat grafts has recently attracted attention worldwide. Obesity as a state characterized by excess fat tissue accumulation is associated with different kinds of cancers. As we know fat tissue is a heterogeneous compound composed of mature adipocytes, ASCs, macrophages, monocytes, and many more cell types with each of them acting in a specific way. According to this, the underlying mechanisms ultimately promoting tumorigenesis are of complex nature. Next to adipose tissue accumulation, obesity also entails a chronic low-grade inflammation with macrophage plus monocyte infiltration and release of cytokines. Cytokines secreted by adipose tissue, also called adipokines, play a role in physical cell signaling and homeostasis and are responsible for maintaining the low-grade inflammation in adipose patients. Interestingly, adipokines exhibit pivotal function in tumorigenesis as well. Adiponectin, a famous representative of the adipokines, is secreted by adipocytes and correlates to the pathogenesis of obesity, diabetes mellitus, and importantly also malignomas. While different studies show a rather inhibitive role of adiponectin, leptin, another cytokine secreted by adipocytes, showed poor prognosis with increasing intratumoral and serum leptin levels [32–34]. Other clinical and experimental studies underline the importance of leptin in the promotion of malignant tumors [35]. Adiponectin, leptin, and many more adipokines are primarily secreted by mature adipocytes, but factors produced and released by the stromal vascular fraction (SVF) equally contribute to tumor progress [36]. ASCs as part of the SVF are multipotent stem cells and support tumorigenesis not only by their ability to release cytokines but also to differentiate into numerous cell lineages, such as endothelial cells which again form blood vessels. As mentioned earlier, adipose tissue in obesity remains in a state of a chronic low-grade inflammation and is thus enriched with macrophages and monocytes. It is hypothesized that these cells also participate in the invasion of tumor stroma, storage, and secretion of carcinogenic factors [36].
To grant tissue vascularization a sufficient network of blood vessels is essential. Proangiogenic cytokines such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), or adiponectin mediating the formation of blood vessels are also secreted by adipose tissue [9, 36]. Responsible for regulated angiogenesis in healthy fat tissue and rejuvenation in the recipient site of lipografts, secreted proangiogenic factors, on the other hand, might as well fuel malignant cells and enable tumor progress by expansion of the vascular system. In vitro and in vivo studies demonstrated the angiogenic capacity of adipose-derived precursor cells.
Another important but not fully understood mechanism is the regulation of the extracellular matrix by matrix metalloproteinases (MMP) which orchestrate the remodeling of the stroma surrounding fat tissue and tumor, respectively. Motrescu et al. [37] postulate that MMP-11 negatively regulates adipogenesis by dedifferentiation which in turn causes accumulation of fibroblast-like cells favoring tumor survival and progression. This process is regulated by a delicate cross-talk between cancer cells and surrounding adipose tissue. Desmoplasia is additionally promoted by secretion of extracellular matrix molecules by adipose stromal cells, which promote tumor growth by shaping the tumor environment [38].
Furthermore, tumor progression could be promoted by homing of ASCs or adipose endothelial cells to the site of the tumor through recruitment of circulating cells. In animal studies Zhang et al. showed migration of labeled, distant adipose tissue cells into the tumor through their entry into systemic circulation [39]. Tumor vascularization and growth by circulating ASCs is a coherent concept and encouraged by a publication of Bellows et al. [40], who observed a correlation of the body mass index (BMI) and the level of circulating progenitor cells.
So far, the role of adipose tissue in tumor formation and the increased risk of tumor formation in obese patients have been illustrated. But how does this stand in conflict with the lipofilling procedure? The idea of suspecting body-own tissue to be carcinogenic after the transfer from one part to another part of the body is indeed intriguing. But considering the fact that lipoaspirates are commonly manipulated by fat graft processing steps and put into an entirely new context by its transfer to a new recipient area without the physiologic provision of nutrients and blood perfusion, this assumption is not unsubstantiated. The transfer of large tissue flaps such as DIEP or TRAM flaps for breast reconstruction after tumor resection with its own vasculature did not show an increased risk of breast cancer relapse.
A case report about a late osteosarcoma recurrence after lipofilling quickly gained attention. Ten years after full remission of a telangiectatic osteosarcoma of the proximal humerus in a 17-year-old female patient, autologous fat was transplanted to treat contour deformities after an initial reconstruction with a vascularized scapular graft [41]. Eighteen months after the lipofilling procedure, a relapse of the previously excised osteosarcoma was diagnosed. To examine this unexpected observation, the authors conducted additional experimental studies showing that adipocytes and ASCs promoted osteosarcoma cell growth in mice. Similar to the observations of Perrot et al., multiple in vitro and in vivo studies revealed the carcinogenic effect of fat grafts. Martin-Padura et al. demonstrated that adipose tissue is rich of endothelial progenitor cells (EPC) contributing to tumor growth. The coinjection of isolated adipose-derived EPCs from lipoaspirates and breast cancer cells significantly increased tumor growth and metastasis. The authors therefore concluded “that caution is warranted in the clinical use of lipotransfer-derived WAT cells for breast reconstruction in patients with breast cancer” [42]. The carcinogenic effect of ASCs was described by Muehlberg et al. [43], who reported increased growth of murine breast cancer cells when murine ASCs were coninjected. Importantly, the growth of human breast carcinoma cells was also stimulated by coinjection with human ASCs in the same mouse line. Zimmerlin et al. postulated after performing cocultivation experiments with human metastatic pleural effusion (MPE) with ASCs that only the growth of active cancer cells is stimulated by ASCs whereas resting cancer cells are not affected [44, 45]. Many more experimental studies reported comparable observations [46, 47]. As a consequence to the ongoing uncertainties, the French Society of Plastic, Aesthetic and Reconstructive Surgery (SOFCPRE) decided in 2007 to “not support fat grafting in a native breast for breast augmentation or following lumpectomy or tumorectomy” until further, extended research results are available granting the safety of lipofilling [48]. The lipofilling of breast cancer patients was only suggested in those after total mastectomy with no breast parenchyma left.
As the SOFCPRE suggested, numerous workgroups dedicated their research to this particular question in the following years mainly focusing on the recurrence of breast cancer. The biggest clinical trial was carried out by Delay et al. [13], who followed up 880 procedures of lipografting to the breast and did not observe any increased risk of tumor recurrence or formation of new tumors. A study including 137 patients who underwent lipofilling after cancer-related mastectomy did not reveal increased breast cancer risk during a median follow-up time of 7.6 years [49]. Similarly, a large multicenter study by Petit et al. assessing 646 lipofilling procedures after breast cancer resection did not show an increased risk of tumor recurrence, and so the authors hypothesized that no explicit contraindication against lipofilling procedures in post breast cancer patients was given [50]. Further clinical trials confirmed that autologous fat transplantation did not lead to cancer recurrence or the formation of new malignancies as expected by the experimental studies mentioned earlier. The only exception is intraepithelial neoplasias. In a matched-cohort study also performed by Petit et al. [51




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








