Augmentation Mammaplasty: Principles, Techniques, Implant Choices, and Complications
Steven Alan Teitelbaum
INTRODUCTION
Breast implants have been FDA (Food and Drug Administration) regulated since the Medical Device Act of 1976. The FDA uses the total reoperation rate as a critical index when evaluating these devices, which has directed patients and surgeons to focus on the pivotal decisions that affect this rate. Reoperation rates at 3 years have consistently been in the 15% to 20% range, and the indications for reoperations have also been consistent over three decades. These statistics suggest that it is not the devices themselves that are responsible for the high reoperation rate. Rather, the problem is the way surgeons use them, including patient education, device selection, surgical planning, and the conduct of the operation. The FDA regulates the sale of implants by a manufacturer, but not the practice of medicine.
Focusing on the avoidance of complications is an ethical imperative, especially for an elective procedure. The same decisions and processes that reduce complications also predictably deliver superior aesthetic results. The modern breast augmentation prioritizes avoiding complications, reducing reoperations, and minimizing iatrogenic damage to breast tissue.
The success of an operation can only be improved when objective endpoints are defined before surgery. For oncologic surgeons and patients it may be local recurrence. For breast augmentation, the only valid quantifiable endpoint is the reoperation rate (when such criteria are defined preoperatively). What local recurrence is to cancer surgeons and patients, reoperation rate is to the aesthetic breast surgeons and patients.
THE PROCESS OF BREAST AUGMENTATION
Surgeons and patients tend to focus on the operation itself as the event that determines the surgical outcome, with preoperative discussion and postoperative management considered to be of secondary importance. This approach fails with breast augmentation where it has been demonstrated that reoperations can be reduced and patient satisfaction increased when a defined process is applied to breast augmentation.1,2,3
Each step of a breast augmentation is no better than the one that preceded it: planning is dependent on patient education; the operative procedure is dependent upon the operative plan; recovery is dependent on the surgical procedure; and final patient satisfaction is the cumulative result of all of these steps. Most important of all is how perceptions of success after surgery were defined at the initial steps of education. Educating patients and having them sign off that their breasts will not match, that some ptosis can be normal, that the only truly natural breasts are non-augmented breasts, that implants can be felt, that no cup size can be promised, and that implant edges or the implant shell may become visible over time can actually increase satisfaction and reduce requests for reoperations. These issues must be made clear to patients as a part of informed consent.
PHILOSOPHICAL APPROACHES TO BREAST AUGMENTATION
There are two schools of thought in breast augmentation: “Give the patient the size she requests” and “Give the patient the size that fits within her breast tissues.” The former assumes that augmentation is a purely cosmetic procedure initiated by the patient. The surgeon’s role is to safely deliver the result she requests, including issues such as the size and type of the implant, incision, and so on.
The latter emphasizes that augmentation is real surgery and that the plastic surgeon must make medically prudent decisions. Patients do not understand which implant will fit within their breast tissues. They do not necessarily understand the consequences of an excessively large implant on the shape of the breast in the short term, nor the adverse effects of them on breast tissues over time.
Neither philosophy should totally trump the other. Both must be considered concurrently and conflicts will arise. Patients may prefer one scar yet the surgeon realizes an objective benefit of another incision; a patient may want an implant of a certain size yet the surgeon may believe it is much too large or small for her breast envelope.
THE CAUSES OF REOPERATION
The only unequivocal endpoint assessing the quality of breast augmentation is the revision rate. Fortunately, the steps that reduce reoperations also create more beautiful breasts. The opposite of a malpositioned implant is an ideally situated implant; the opposite of a contracted capsule is a soft capsule, and so on.
CAPSULAR CONTRACTURE
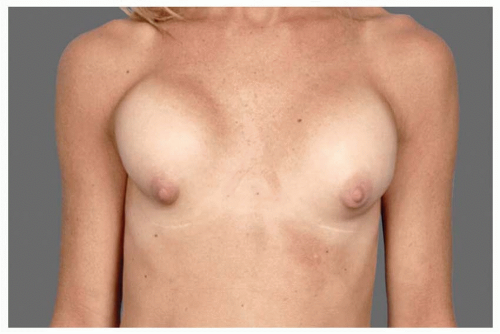
Capsular contracture is and has always been a leading cause of revisions. As scar tissue thickens and tightens around the implant, the breast feels firmer, it looks becomes more spherical, the implant migrates superiorly, and the breast can be painful. Though patients may say, “my implants got hard,” in fact, the implants are soft but constrained within a tightening envelope of their own tissue (see Figure 53.1).
The proximate cause of capsular contracture is inflammation, which in turn can be caused by silicone gel bleed, glove talc, blood, tissue trauma, and bacteria. Current evidence supports Staphylococcus epidermidis biofilm as a significant cause of capsular contracture.4,5 Data include the association of biofilm with contracted capsules, the experimental induction of capsular contracture through inoculation of breast implants with Staph epidermidis, and reduction in capsular contracture from the use of antibiotic irrigation.
Breast augmentation is a “clean-contaminated” case because there are bacteria within the breast, the concentration of which is highest in the area of the periareolar (PA) incision. At least one study has shown a statistically significant increase in the percentage of capsular contracture using the PA approach.6 (see Figure 53.2).
Patients should be educated before surgery that it is normal to feel the capsule around the implant (Baker grade II), that the capsules on the two sides never develop equally, and that revision should only be considered for a Baker grade III (firm and distorted) or Baker grade IV (painful). Surgery is not indicated for a Baker II capsule because there is little likelihood of creating and maintaining a Baker I (no discernable capsule).
While saline implants had an advantage in reducing capsular contracture over older generation silicone gel implants, the advantage no longer exists over today’s silicone implants perhaps due to shells that reduce silicone diffusion or the use of a silicone filler with fewer impurities.7,8 Meta-analyses demonstrate the benefit of implant texturing in the subglandular position, but no such advantage is seen in the submuscular position.
Malposition
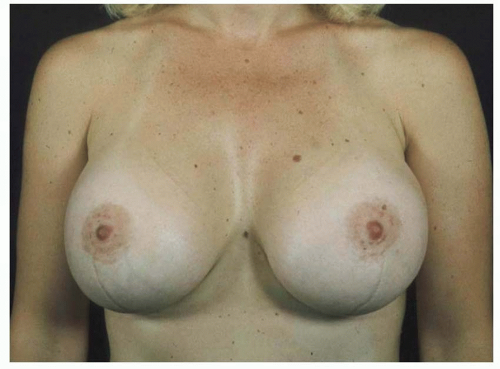
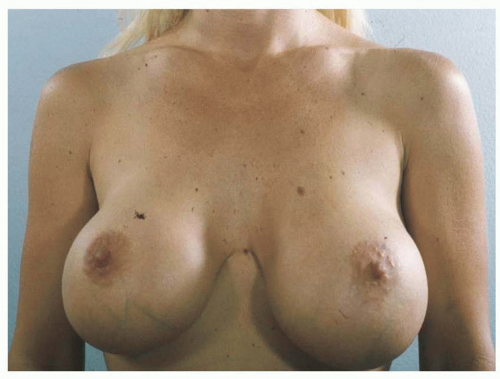
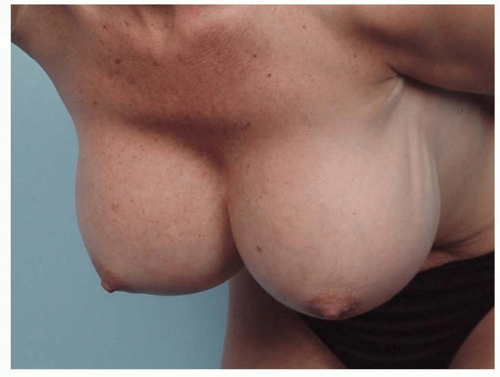
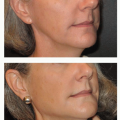
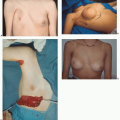
Implant malposition creates some of the most severe deformities following breast augmentation (Figures 53.3, 53.4, 53.5 and 53.6). Breast appearance is determined by the amount and distribution of volume, which in turn is determined by the position of the breast implant.
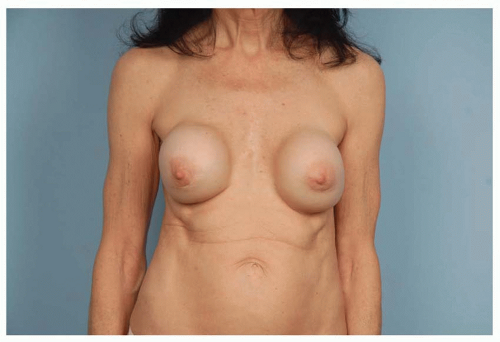 FIGURE 53.1. As scar tissue thickens and tightens around breast implants, the shape becomes more spherical, frequently rises upward, becomes firm, and often painful. |
 FIGURE 53.3. Inferior malposition results in up-pointing nipples and an empty upper pole. Note the position of the inframammary scars above the current inframammary folds. |
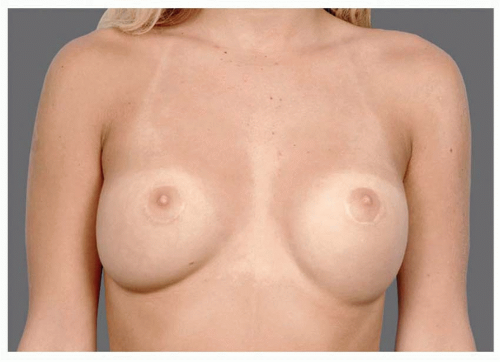 FIGURE 53.4. Lateral malposition results in an underfilled medial meridian of the breast and a widened intermammary distance. |
Over-dissection allows an implant to move out from its ideal position and incomplete dissection prevents an implant from settling in its ideal position. The use of pressure wraps, circumferential bands, and special bras to push the implant against undivided tissues or to prevent an implant from migrating into an over-dissected space is ineffective. Accurate dissection is more effective than any external influence.
Excessive lateral or inferior dissection allows implants to malposition in those directions; division of the pectoralis major muscle along the sternum risks symmastia and window-shading of the muscle which reduces coverage and leads to animation deformity; incomplete division of the pectoralis major muscle along the medial inframammary fold (IMF) predisposes to superior and lateral malposition; and failing to divide accessory pinnate origins of the pectoralis just lateral to its main trunk along the lateral sternal border may cause lateral malposition (or restrict ideal medial position of the implant and fill in that area).
Inferior and lateral over-dissection is most often inadvertent, medial over-dissection is often intentionally done to gain more cleavage. Not only does this reduce muscle coverage over the implant where tissue is thinnest, but also this method is least effective in the situations where medial fullness is most desirable: pectus carinatum, a wide intermammary distance, or extreme tightness of the skin against the sternum. Implant edge visibility and traction rippling caused by excessive medial dissection or division of medial pectoralis attachments along the sternum are largely uncorrectable deformities.
 FIGURE 53.6. With medial malposition, the lateral breast is underfilled and the nipple points outward. Presternal skin can be raised off of the sternum. |
Even with a precise pocket, gradual migration from weight, pressure, and gravity can occur, particularly with chest wall deformities. For instance, a pectus excavatum will increase the likelihood of medial migration (symmastia), and a carinatum shape will predispose a patient to lateral malposition (Figures 53.7, 53.8 and 53.9).
Whenever the musculocutaneous fibers that define the perimeter of the breast are divided, then it is the tenacity of the neighboring tissues that will either hold the implant in place or allow it to passively migrate. In order to create an ideally proportioned breast, the IMF may need to be lowered, but it must be done precisely; random and blunt lowering of the IMF is unpredictable and uncontrolled.
A surgeon should assess the strength of attachments between the soft tissues and chest wall at the inferior edge of the IMF incision. When these attachments are weak, the inferior cut edge of scarpa’s fascia can be sewn to the muscle fascia. Some surgeons will routinely place such sutures with the inframammary incision.
 FIGURE 53.7. Worm’s eye view of patient with lateral implant malposition on the left and capsular contracture on the right. |
Size Exchange
Reoperation to change the size of an implant should be rare. Except for unusual changes in weight or lifestyle, reoperations for size exchange are usually the result of inadequate patient education and implant selection.
Determining implant size with bags of rice, water, and implants; filling a larger bra; prediction of a cup’s size; or looking at photos puts the patient into a mind-set that implant size is totally her choice. This allows the patient to reconsider size in the future. When patients are educated to choose the implant that ideally fills their breasts based upon their breasts’ dimensions, then future rationale for changing the size is limited. In addition, allowing a patient to expect to be a particular bra size is misleading because there is no standard for bra sizing.
When surgeons determine the implant size intraoperatively, they may find themselves being criticized by a patient dissatisfied with their size. This can be avoided when the implant size is agreed upon preoperatively. It is also apparent that sitting a patient up during surgery with air around her implants as swelling begins is not as accurate or predictive as preoperative objective tissue measurements (and prolongs operative time, increases tissue trauma, bleeding, and raises the possibility of contamination).
There is a misunderstanding that using measurements makes implant selection a surgeon’s choice. Implant size is as much as patient’s choice when she chooses to tell the surgeon to select the size that is best for her tissues as when she chooses to tell the surgeon a specific size. Optimal patient education and informed consent teaches patients the evidence-based benefits of published measuring systems, and the patient then chooses to use those systems to determine implant size. Patients are taught that breasts fill from the bottom up as if sand were being poured in from a funnel. There is an ideal volume to fill any particular breast. If the volume is excessive, the upper pole will be too full, and if the volume is insufficient, the upper pole will remain underfilled.
There is also an adage to “go larger, because patients always wished they were bigger.” This is false. Many patients do request a second operation to receive smaller implants, and the patients who think they are too big often have soft tissue coverage and stretch problems as a result of those large implants. Those who feel too big often suffer from anguish or embarrassment, while those who contemplate being larger are not distraught, but perhaps just want “more of a good thing.” But when the concept of “the right size” is taught to patients, then future requests for size exchange are nearly eliminated.
Ptosis
Ptosis recognized after breast augmentation either preceded the augmentation or was created by it. Preexisting ptosis may have been unrecognized or the patient may have declined having a mastopexy. Either way, augmented ptotic breasts are inevitably misshapen, and the weight and pressure of the implants can make the ptosis worse. Implants in thin, ptotic, and empty envelopes have a high tendency for palpable folds.
Breast augmentation is not a treatment for breast ptosis. An implant can fill an empty breast, but it does not raise nipples or shorten a long N:IMF (nipple to inframammary fold) distance. If a patient has a preoperative N:IMF of >9 cm, a mastopexy should be considered; when N:IMF > 10 it is required (Chapter 54).
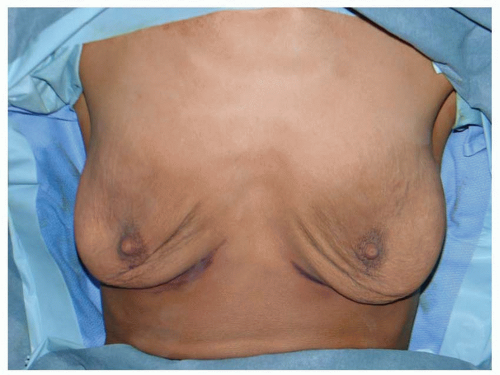
Many patients with ptotic breasts do not want a mastopexy. In an effort to avoid the mastopexy, a very large implant might be used, perhaps making the breasts larger than what the patient desires. In some cases, the bottom of the implant remains at the IMF, and the breast tissue descends off the front of the implant mound, creating a down-pointing nipple and an upper bulge. In other cases, the implant falls to the bottom of the breast envelope and creates even more lower pole stretch, leading to more upper breast emptiness and an upturned nipple (see Figures 53.10, 53.11, 53.12 and 53.13). If a ptotic patient refuses mastopexy, then she should not have an augmentation. This is one of the most frequent avoidable errors in breast augmentation.
If a breast has a large envelope by dimensions and a small amount of existing parenchyma, (See “Size” on page 11), then it may take a large implant to fill the breast. Yet such skin may stretch from the pressure and weight of the large implant.
Post augmentation ptosis is invariably related to problematic patient tissues. The N:IMF distance on maximal stretch is an indication of the amount of skin between the nipple and the fold. When an implant is placed, the breast fills from the bottom up. If this distance is short, a given sized implant will create more upper bulge; if this distance is long, then a similarly sized implant will remain in the lower pole. Anterior pull skin stretch (APSS) is measured by pulling on the skin just medial to the areola and determining how far forward it will move with gentle pressure. When it is short it indicates that the skin envelope has little stretch to accommodate an implant, and when it is long it indicates that the skin will not lay tight against the implant. Parenchymal contribution to stretched envelope fill (PCSEF) is a measurement of how full a breast already is. If a given implant is put into a breast that is already full, it will result in more upper fullness and definition of the implant, whereas an empty breast will have more room to accommodate the implant. When N:IMF > 9.5, APSS > 4, and PCSEF < 20%, the surgeon must recognize that the patient is in a problematic situation and is at risk for post augmentation ptosis that will require a revision surgery (see upcoming section and illustrations of these measurements).
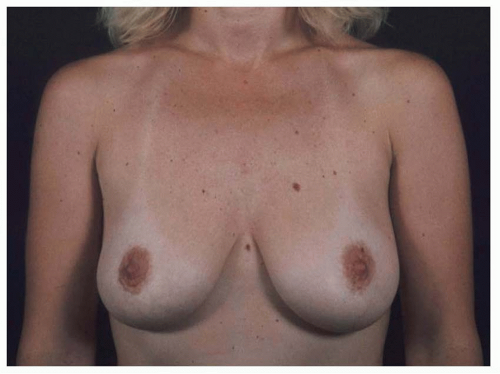 FIGURE 53.10. Postpartum with a base width of 15 cm and an N:IMF of 11 cm. Her breasts are heavy and ptotic, but she did not want a mastopexy. |
Shell Failure
Retrieval studies demonstrate that over half of all shell failures are due to sharp instrument injury during implantation. Even a small scratch increases the chance of shell failure in stress cycle testing.
The implant should be kept in its thermoform packaging and touched only by the surgeon after changing into new gloves. Saline implants can be rolled and placed through smaller incisions than silicone. Since they are filled after insertion, all sizes of saline implants can be placed through an incision of the same length. Large, textured, or highly cohesive breast implants require longer incisions. There is no consistent rule about incision length. Incisions should be of adequate length to assure atraumatic insertion of the implant, with no abrasion to skin edges, damage to the implant shell, or “fracturing” of the fill in the case of some highly cohesive implants. Breast implants are most safely inserted through incisions with a minimal length of 4 to 4.5 cm, with longer incisions required for implants with a base width over 13 cm or a volume over 350 cc. While IMF incisions can be lengthened, staying within a small areola or axilla can make it challenging to use those incisions for gentle implant insertion.
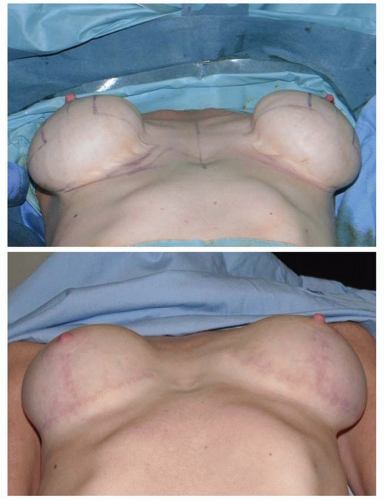 FIGURE 53.13. Top: two years after augmentation with subglandular implants in a patient who declined mastopexy. Note severe damage to the skin at periphery of implant and markings for a mastopexy. |
Cautery and needles must never be in proximity to the implant. Pocket adjustment after implant placement must be made with retractors designed for implants. The surgeon should develop a system for closure that creates exposure and protects the implant from the needle.
Underfilled saline or silicone implants are more subject to collapse, shell folding, and possible failure along folds. Surgeons should use implants with a fill that optimizes shell folding, which is a consequence of both fill volume and fill cohesivity. Intraluminal betadine in saline implants can lead to shell delamination. Betadine irrigation into the pocket for both saline and silicone gel implants is against all manufacturers’ Directions For Use. But given its value in reducing capsular contracture, this prohibition may be reviewed.
Rippling
Rippling in the décolletage inhibits the ability to wear lowcut clothing. Palpability reduces confidence and feels odd. Rippling is probably the most distressing of all breast implant issues for patients. It is the least likely of all secondary augmentation deformities to be corrected (or even prevented) if the breast tissue, muscle, or skin is thin, loose, or damaged (Figures 53.14, 53.15, 53.16 and 53.17).
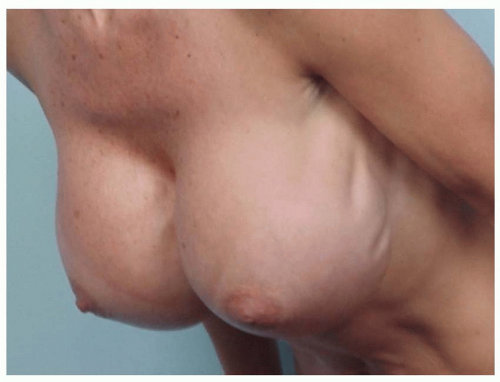 FIGURE 53.14. Implant underfill rippling can be visible with the patient upright, but is exacerbated bending forward. Adequate tissue coverage can conceal underfill rippling. |
There are two types of rippling: implant underfill rippling and traction rippling.
Implant underfill rippling occurs when an implant shell is filled to a volume that does not prevent upper shell collapse with the patient upright, allowing shell folding as the filler descends to the dependent portion of the implant. Just as there is an ideal fill volume for the breast, there is an ideal fill volume for the shell. The manufacturer (or surgeon filling a saline implant) must balance the advantages of lower fill volume (less roundness and more softness) versus higher fill (less rippling). Increased cross-linking of silicone polymers increases cohesivity, which may reduce rippling by decreasing the amount of inferior descent of fill material within the shell.
Underfilled implant rippling can be camouflaged if tissue thickness over the implant is adequate, but the implant shell is nevertheless rippled. Even highly cohesive filler implants that are underfilled can cause rippling by pulling on thin overlying tissues.
Some surgeons opine that textured implants ripple more than smooth implants. Textured shells are only slightly thicker than smooth shells. Perhaps this stiffens the shell enough so that folds less readily dissipate upon light palpation.
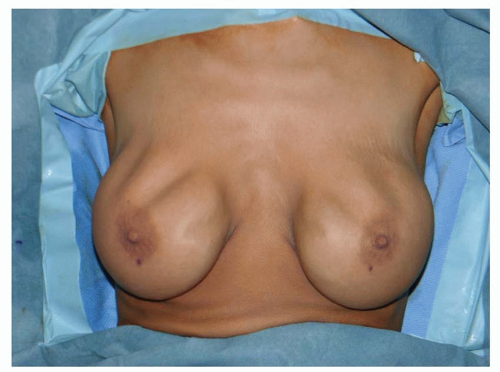 FIGURE 53.16. Severe underfill rippling in a nulliparous 26-year-old, only 4 years after augmentation with high-profile saline implants. |
Traction rippling occurs when an implant pulls on the capsule, which in turn pulls on the skin, much like a heavy object in a shirt pocket would create folds in the fabric.
Longstanding high-profile or contracted implants can create bowl-shaped deformation of the rib cage allowing the anterior surface of the implant to collapse and ripple.
Breasts most prone to visible rippling are those with inadequate tissue coverage (e.g., when pinch thickness of the skin and subcutaneous tissue superior to the breast parenchyma is less than 2 cm) or when pinch thickness at the IMF is less than 0.5 cm. Breasts with preexisting ptosis and those that are susceptible to postoperative ptosis (APSS > 4, NIMF > 9, and PCSEF < 20%) are also prone to rippling. These situations should be identified preoperatively. No type of breast implant can compensate for inadequate tissue coverage, and deformities that occur are largely uncorrectable. Surgeons should consider refusing to augment breasts when such tissue problems are significant. The role of tissue coverage in preventing rippling cannot be overstated. Therefore, the priority at primary augmentation is to maximize coverage and avoid tissue damage.