Augmentation Mammaplasty: General Considerations
G. Patrick Maxwell
Matthew B. Baker
Allen Gabriel
Introduction
Glandular hypomastia may occur as a developmental or involutional process and affects a significant number of women in the United States. Developmental hypomastia is often seen as primary mammary hypoplasia or as a sequela of thoracic hypoplasia (Poland syndrome) or other chest wall deformity. Involutional hypomastia may develop in the postpartum setting and may be exacerbated by breast-feeding or significant weight loss. When compared to the norm, inadequate breast volume may lead to a negative body image, feelings of inadequacy, and low self-esteem (1). These disturbances may adversely affect a patient’s interpersonal relationships, sexual fulfillment, and quality of life (2).
There has been a steady increase in breast augmentation surgery with the emerging importance of body image, changes in societal expectations, and the increasing acceptance of aesthetic surgery in the United States. Augmentation mammaplasty was performed 355,671 times in 2008 as the most frequently performed cosmetic surgical procedure in women in the United States (3). This is the first time that breast augmentations procedures have surpassed liposuction. In this chapter, we review the history of breast augmentation, operative planning and technique, and some perioperative and late complications of the procedure. The evolution of modern breast implants is described, and the controversy surrounding the use of silicone gel in breast augmentation is discussed.
History
The first report of successful breast augmentation appeared in 1895 in which Czerny described transplanting a lipoma from the trunk to the breast in a patient deformed by a partial mastectomy (4). In 1954, Longacre described a local dermal-fat flap for augmentation of the breast (5). Eventually, both adipose tissue and omentum were also used to augment the breast. However, the clinical results of using autogenous tissue for breast augmentation were often unpredictable and unacceptable (6).
During the 1950s and 1960s, breast augmentation with solid alloplastic materials was carried out using polyurethane, polytetrafluoroethylene (Teflon), and expanded polyvinyl alcohol formaldehyde (Ivalon sponge). Ultimately, the use of these materials was discontinued after patients developed local tissue reactions, firmness, distortion of the breast, and significant discomfort (7). Various other solid and semisolid materials have been injected directly into the breast parenchyma for augmentation, including epoxy resin, shellac, beeswax, paraffin, petroleum jelly, and liquid silicone. Liquid silicone (polydimethyl siloxane) was originally developed in the aeronautics industry during World War II. In 1961, Uchida reported the injection of liquid silicone into the breast for breast augmentation (8). Unfortunately, injection of liquid silicone resulted in frequent complications, including recurrent infections, chronic inflammation, drainage, granuloma formation, and even necrosis (9,10). Breast augmentation by injection of free liquid silicone was abandoned in the United States in light of these complications.
The modern breast implant is a two-component prosthetic device manufactured with a nearly impermeable silicone elastomer shell filled with a stable filling material, consisting of either saline solution or silicone gel. This shell plus filler implant was originally developed by Cronin and Gerow in 1963 using silicone gel as the filling material contained within a thin, smooth silicone elastomer shell (11). Since that time, both silicone gel– and saline-filled implants have undergone several technical alterations and improvements.
Breast Implant Evolution
Saline-Filled Implants
The use of inflatable saline-filled breast implants was first reported in 1965 by Arion in France (12). The saline-filled implant was developed in order to allow the noninflated implant to be introduced through a relatively small incision, followed by inflation of the implant in situ (13). Although the incidence of periprosthetic capsular contracture was lower with the saline-filled implants compared to the earlier generation of silicone gel–filled implants, the deflation rate was initially quite high. The original saline-filled implants manufactured by Simiplast in France had a deflation rate of 75% at 3 years and was subsequently withdrawn from the market. In 1968, the Heyer-Schulte Company introduced its version of the inflatable saline-filled breast implant (Mentor 1800) in the United States.
The thin, platinum-cured shell and the leaflet-style retention valve were two features of the early saline-filled implants that contributed to their high deflation rate (14). The silicone elastomer shell of the saline-filled implant has been improved by making it thicker and by employing a new room-temperature vulcanization process. This process is used in the manufacture of all saline-filled implant shells currently available from Allergan (formerly Inamed Corporation and McGhan Medical) and from Ethicon [formerly Mentor Corporation (which acquired Heyer-Schulte)]. The original Heyer-Schulte saline-filled implant shell had a leaflet-style retention valve through which the implant was inflated (15). A more reliable diaphragm valve was developed and is currently incorporated into the shell of all modern saline-filled breast implants.
Table 106.1 Saline-filled Breast Implants | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Saline-filled implants are manufactured with a range of recommended fill volumes. Mild breast asymmetry may be corrected by taking advantage of this range of recommended fill volumes during placement of the implants. Underfilling saline-filled implants may lead to increased deflation rates due to folding or friction subjected to the implant shell and is not recommended. Underfilling saline-filled implants may also lead to a wrinkled appearance or rippling with the breast in certain positions. Saline-filled implants have historically performed better when slightly overfilled and when placed under thicker soft tissue coverage. Although these implants may be slightly overfilled, aggressive overfilling may lead to a more spherical shape and scalloping along the implant edge with knuckle-like palpability and unnatural firmness. Another potential disadvantage of saline-filled implants is that the consistency of these implants on palpation is similar to that of water instead of the more viscous feel of natural breast tissue. Several saline-filled breast prostheses are available from both corporations with different surface textures, shapes, and degrees of projection (Table 106.1).
Silicone Gel–Filled Implants
Silicone Chemistry
Silicone is a mixture of semi-inorganic polymeric molecules composed of varying-length chains of polydimethyl siloxane [(CH3)2–SiO] monomers. The physical properties of silicones are quite variable, depending on the average polymer chain length and the degree of cross-linking between the polymer chains (16). Liquid silicones are polymers with a relatively short average length and very little cross-linking. They have the consistency of an oily fluid and are frequently used as lubricants in pharmaceuticals and medical devices. Silicone gels can be produced of varying viscosity by progressively increasing the length of the polymer chains or the degree of cross-linking. The consistency of silicone gels may vary widely from a soft, sticky gel with fluid properties to a firm, cohesive gel exhibiting shape retention or form stability, depending upon the polymer chain length and the degree of cross-linking. Extensive chemical cross-linking of the silicone gel polymer will produce a solid form of silicone referred to as an elastomer with a flexible, rubber-like quality. Silicone elastomers are used for the manufacture of facial implants, tissue expanders, and the outer shell of all breast prostheses. The versatility of these compounds has made them indispensable in aerospace engineering, medical devices, and the pharmaceutical industry.
Evolution of Silicone Gel–Filled Implants
The first generation silicone gel–filled implant was introduced in 1962 by Cronin and Gerow and was manufactured by Dow Corning Corporation (11). The shell of the first-generation implant was constructed using a thick, smooth silicone elastomer as a two-piece envelope with a seam along the periphery (Table 106.2). The shell was filled with a moderately viscous
silicone gel. The implant was anatomically shaped (teardrop) and had several Dacron fixation patches on the posterior aspect to help maintain the proper position of the implant. Unfortunately, these early devices had a relatively high contracture rate that encouraged implant manufacturers to develop second-generation silicone gel–filled implants.
silicone gel. The implant was anatomically shaped (teardrop) and had several Dacron fixation patches on the posterior aspect to help maintain the proper position of the implant. Unfortunately, these early devices had a relatively high contracture rate that encouraged implant manufacturers to develop second-generation silicone gel–filled implants.
Table 106.2 Evolution of Silicone Gel–filled Breast Implants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Table 106.3 Silicone Gel-filled Breast Implants | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the 1970s, the second-generation silicone implants were developed in an effort to reduce the incidence of capsular contracture with a thinner, seamless shell and without Dacron patches incorporated into the shell. These implants were round in shape (nonanatomic) and filled with a less viscous silicone gel to promote a natural feel. However, the second-generation breast implants were plagued by diffusion or bleed of small silicone molecules into the periprosthetic intracapsular space due to their thin, permeable shell and low-viscosity silicone gel filler. This diffused silicone may be encountered as an oily, sticky residue surrounding the implant within the periprosthetic capsule during explantation of older silicone-filled implants. Microscopic silicone particles have been shown to be present within the periprosthetic tissues and even within the draining lymphatics and axillary lymph nodes (17). The phenomenon of silicone bleed has not been shown to create significant local or systemic problems (18). However, long-term device failure issues have plagued these second-generation devices due to the thin, weak shell composition.
The development of the third-generation silicone gel-filled implants in the 1980s focused on improving the strength and integrity of the shell in order to reduce silicone gel bleed from intact implants and to reduce implant rupture and subsequent gel migration. The formerly Inamed Corporation (now allergen) developed a multilayer implant shell in which a patented barrier-coat material is sandwiched between two layers of silicone elastomer (Intrashiel). The formerly Mentor Corporation now ethicon also developed a shell for their silicone gel–filled breast implants, which consists of a multilayered silicone elastomer. These third-generation prostheses reduced gel bleed to an almost immeasurable level and significantly lowered device shell failure rate.
After the Food and Drug Administration (FDA) required the temporary removal of third-generation silicone gel implants from the U.S. market in 1992, the fourth-generation gel devices evolved for their market reintroduction. These silicone gel breast implants were designed under more stringent American Society for Testing Methodology and FDA-influenced criteria for shell thickness and gel cohesiveness. Furthermore, the fourth-generation devices were manufactured with improved quality control and with a wider variety of surface textures and implant shapes (Table 106.3).
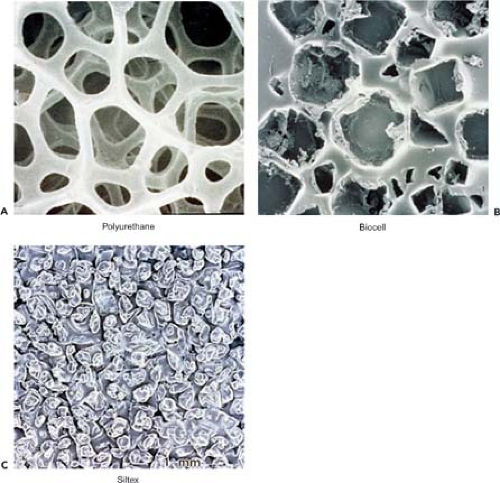
The development of the anatomically shaped fourth generation of gel implants was based on recognition that breast augmentation must account for the individual patient’s breast shape and chest wall dimensions in order to produce the most natural result. The shells of anatomically shaped breast implants are manufactured with a textured surface to encourage ingrowth and disorganization of the periprosthetic scar tissue and to reduce the incidence of implant rotation and resulting breast deformity (19). Figure 106.1 shows micrographs of the textured surface of several implants.
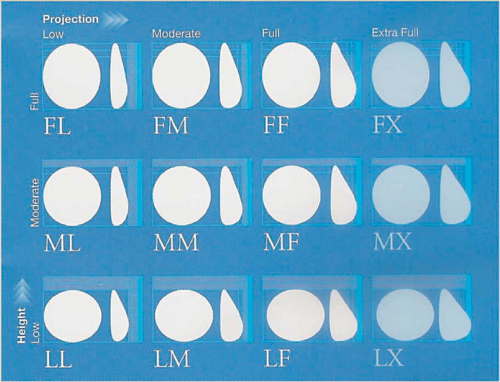
With the evolution of the fifth-generation silicone gel implants, the concept of anatomically shaped implants was carried to the next level. The BioDimensional Planning System in which a matrix of 12 possible combinations of implant height and projection was introduced for the specific needs of the individual patient. These anatomically shaped (style 410) implants are available with a range of volumes and any of the 12 combinations of low, moderate, and full height with low, moderate, full, and extra projection (Fig. 106.2). The Contour Profile Gel (CPG) implant has been designed by the Mentor Corporation with a more rounded and projecting lower pole and a flatter, more sloping upper pole to yield a more natural breast shape in breast augmentation and reconstruction.
It is believed that as the gel flows inferiorly with gravity, the upper portion of the implant collapses due to a relatively decreased volume in the upper pole. To combat this characteristic of silicone gel implants, efforts have been made to develop fifth-generation devices containing more cohesive silicone gel that exhibits less flow and more form stability. The development of these fifth-generation devices has resulted from advances in the technology of silicone gel.
The Approval of Silicone Gel Implants
In 1976, the U.S. Congress passed the Medical Device Amendment to the Food, Drug, and Cosmetic Act, which gave the FDA authority over implantable medical devices (14). Breast implants that were currently available at that time or those that were “substantially equivalent” were allowed to remain in use until the FDA could formally review their safety and efficacy. In 1988, the FDA called for the manufacturers of silicone gel–filled implants to submit Pre-Market Approval applications providing data that could substantiate the claim of safety and efficacy for these devices. In November 1991, the FDA convened an advisory panel of experts to evaluate the manufacturers’ data and to hold public hearings. The advisory panel concluded that more research was needed to establish the safety and efficacy of silicone gel–filled breast implants but that these devices should remain available for use by the general population while the clinical trials were carried out.
Instead of ratifying the panel’s recommendation, the FDA commissioner called for a voluntary moratorium on the use of all silicone gel–filled implants. Further evaluation by the advisory panel led the commissioner to rule in April 1992 that silicone gel breast implants were not necessarily unsafe, but that the law required more data than the manufacturers had supplied to establish the safety and efficacy of these devices (14). Although the news media and the general public regard this moratorium as a ban on the use of silicone gel–filled implants due to their supposed hazardous nature, their use has never been officially banned. From 1992 to 2006, the use of silicone gel–filled implants for aesthetic breast augmentation and breast reconstruction was restricted to patients with breast deformities who participated in clinical trials. Criteria and eligibility for enrollment in the silicone gel–filled breast implant Adjunct Study was developed both by Mentor (now Ethicon) and Inamed (now Allergan) (Table 106.4).
The manufacturers continued to improve their gel-filled products and presented the results of the silicone gel–filled breast implant Adjunct Study to the FDA. On November 17, 2006, the FDA approved the new and improved silicone gel–filled breast implants produced by the two manufacturers for breast reconstruction and for cosmetic breast augmentation. The approval was given with a number of conditions, including
a requirement to complete 10-year studies on women who have already received the implants and a 10-year study on the safety of the devices in 40,000 women. The postapproval studies will be closely monitored and as of the writing of this chapter, both manufacturers have enrolled the minimum required number of patients.
a requirement to complete 10-year studies on women who have already received the implants and a 10-year study on the safety of the devices in 40,000 women. The postapproval studies will be closely monitored and as of the writing of this chapter, both manufacturers have enrolled the minimum required number of patients.
Alternative Filling Materials
Manufacturers of breast implants have developed alternative filler materials in response to concerns about the safety of silicone gel (24). In 1991, the Bioplasty Corporation introduced the Misti Gold implant, which uses polyvinylpryrrolidone (PVP) as the filling material. The PVP filling material is described as a bio-oncotic gel and is believed to be more radiolucent than silicone. NovaMed acquired Bioplasty and still markets the PVP-filled implant under the name NovaGold outside the United States. The PIP Corporation in France developed breast implants filled with a hydrated polysaccharide gel (hydrogel). However, there have been reports of swelling of hydrogel and PVP-filled implants postoperatively due to the osmotic pressure gradient. In December 2000, the British Medical Devices Agency issued an alert citing a lack of studies that demonstrate the safety of these implants. In 1994, the LipoMatrix Corporation developed the Trilucent implant, which was filled with a triglyceride derived from soybean oil (24). However, problems developed with oil bleed, tissue irritation, and a foul, rancid odor. These implants were withdrawn from the market in 1999, and none of the alternative filling material implants are currently available in the United States.
The Absence of Evidence-Based Medicine
Of interest, silicone-containing compounds are ubiquitous in everyday life. The general public has been exposed to them for more than 50 years in consumer products such as hairsprays, suntan lotions, and moisturizing creams. Silicones are extremely resistant to the action of enzymes when implanted into living tissue largely due to their hydrophobic nature (16). This makes silicone compounds extremely stable and inert. Silicones are often used in the consumer safety testing industry as the standard to which all other products are compared for biocompatibility (16). While elemental silicon and silicone particles are detected in periprosthetic tissues, the biologic significance of this finding remains undetermined and uncharacterized (25). In one study, no significant difference was found in levels of antisilicone antibodies between patients who had silicone elastomer tissue expanders and control subjects (26).
Several clinical studies have shown no difference in the incidence of autoimmune diseases in mastectomy patients receiving silicone gel implants compared to patients who had reconstruction with autogenous tissue (27,28,29,30). Even meta-analysis research combining data from more than 87,000 women has revealed no association between silicone breast implants and connective
tissue diseases (31). In the modern era of evidence-based medicine, it seems that the only exception to the rule of science is in the use of silicone gel–filled breast implants, where lawsuits and hysteria supersede science. Notably, virtually all industrialized nations in the world except the United States use silicone gel implants almost exclusively for breast augmentation.
tissue diseases (31). In the modern era of evidence-based medicine, it seems that the only exception to the rule of science is in the use of silicone gel–filled breast implants, where lawsuits and hysteria supersede science. Notably, virtually all industrialized nations in the world except the United States use silicone gel implants almost exclusively for breast augmentation.
Table 106.4 Inclusion Criteria for Participation in the Silicone Gel–filled Breast Implant Adjunct Clinical Study | ||
|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree