Common manifestations
Atypical manifestations
aGVHD
cGVHDa
aGVHD
cGVHD
Maculopapular rash
Poikiloderma
Generalized erythroderma
Lichen planus-like features
Bullae
Sclerotic features
Type II PRP [13]
Morphea-like features
Lichen sclerosis-like features
Papulosquamous lesions
Total body leukoderma [22]
Depigmentation
Wolf isotopic pattern [27]
Alopecia
Hypo- and hyper-pigmentation
Sweat impairment
Dermatomyositis [36]
Ichthyosis
Keratosis pilaris
Erythema
Maculopapular rash
Pruritus
Calcinosis cutis universalis [69]
Bullae [70]
Eczematous Eruptions
Chronic cutaneous GVHD may present with eczematous eruptions [4, 5] and variants such as atopic dermatitis-like [6] and eczematoid GVHD [8]. This manifestation is not associated with a history of atopy in patients or donors. It is characterized by pronounced pruritus and either widespread or limited areas of dry skin, erythema, papules, and perifollicular accentuation. Peripheral eosinophilia and elevated IgE levels are common in cases of atopic dermatitis-like GVHD [6]. Histology demonstrates findings consistent with GVHD in addition to features of eczematous dermatitis in most cases. Atopic dermatitis-like chronic GVHD is associated with a favorable outcome, with a good response to topical therapies such as emollients, topical corticosteroids, and topical tacrolimus, as well as to ultraviolet phototherapy and prednisone, with or without immunosuppressant agents for GVHD [6]. Conversely, eczematoid GVHD, which has been described as a more aggressive variant that often progresses to erythroderma, can be difficult to manage and is associated with a poor prognosis [8]. It is important to note that some have suggested that eczematoid GVHD may be more accurately classified as a variant of late-onset acute GVHD based on precipitating factors and histopathologic findings [80].
There have also been reports of GVHD presenting as eczema craquele in both acute [3] and chronic GVHD [7], with diffuse or localized areas of reticulated erythema on exam (Fig. 12.1), and findings of GVHD and eczema on histology.
Acute cutaneous GVHD presenting as a contact dermatitis has been reported in one case of an infant who developed a hyperemic belt-shaped eruption in the diaper region following allogeneic stem cell transplant [2]. The clinical presentation in this case was progressive and fatal.


Fig. 12.1
Chronic GVHD presenting as reticulated erythema with fine scale mimicking eczema craquelé
Papulosquamous Eruptions
Psoriasiform eruptions have been described in both acute [9, 10] and chronic [1, 4, 5, 11, 12] cutaneous GVHD. Typical skin findings include discrete guttate, annular, or confluent erythematous scaly patches and plaques with micaceous scale that may involve any part of the body, including the scalp, face, hands, and feet [1, 4, 5, 10, 11]. Histology demonstrates features of GVHD as well as psoriasis in most cases. This manifestation is not associated with a history of psoriasis in either donors or patients.
An eruption resembling type II (atypical adult) pityriasis rubra pilaris (PRP) has been described as a very rare variant of acute GVHD, with only one reported case [13]. Clinically, this variant reveals a pattern of PRP with erythematous follicular hyperkeratotic papules coalescing to form diffuse red-orange plaques with islands of unaffected skin, palmoplantar keratoderma, and marked ichthyosiform scaling. Histology shows typical changes of acute GVHD, such as basal vacuolar degeneration, scattered apoptotic keratinocytes at the dermoepidermal junction, and lymphocyte satellitosis adjacent to dyskeratotic keratinocytes, as well as some characteristics of PRP, including psoriasiform hyperplasia, parakeratosis, and follicular plugging. The patient in this report achieved a partial response with treatment [13].
Few cases of pityriasis rosea (PR)-like [14] and inverse PR-like [3] chronic GVHD have been reported (Fig. 12.2). A herald patch preceded the development of a more diffuse eruption in half of the reported PR-like cases [14]. Histopathology in these cases demonstrated features of both PR and GVHD.


Fig. 12.2
A case of chronic cutaneous GVHD mimicking inverse pityriasis rosea
Reactive Erythema
Erythroderma and Exfoliative Dermatitis
Erythroderma and exfoliative dermatitis have been reported as a variant of chronic GVHD [4, 8, 12, 21]. This is often preceded by papulosquamous, morbilliform, or annular eruptions [4]. While exfoliative dermatitis can be seen in progressive acute GVHD, this manifestation does not necessarily reflect a progression of disease in chronic GVHD.
Ichthyosiform GVHD
Ichthyosis is an acknowledged feature of chronic cutaneous GVHD [1]. Acquired ichthyosis has also been reported as a manifestation of acute GVHD and is typically preceded by or associated with other signs of cutaneous and extracutaneous GVHD [15, 16]. A personal or family history of ichthyosis is not felt to be a relevant factor in this presentation.
Extensive Follicular Eruptions in GVHD
Follicular erythema and follicular keratosis or keratosis pilaris-like lesions can be early manifestations of acute and chronic GVHD, respectively. However, a follicular eruption as a major clinical manifestation of acute or chronic GVHD is uncommon (Fig. 12.3). In acute follicular GVHD, diffuse eruptions of erythematous, follicular papules develop early in the course of disease and precede or are simultaneous with the classic morbilliform rash. In some reported cases, the eruption was progressive and persistent, and the patients died shortly after the diagnosis was made; however, because of the small number of reports, it is unknown if acute follicular GVHD indicates a more severe course than other types of acute GVHD [18–20]. In contrast, chronic follicular GVHD develops late in the course of disease and is considered a clinical variant with more favorable prognosis [3, 23–25]. Another variant of chronic follicular GVHD that resembles open and closed acne-like comedones, termed comedonal-GVHD, has been reported in a few patients (Fig. 12.4). Like other forms of chronic follicular GVHD, this variant has been associated with a good clinical outcome [26].

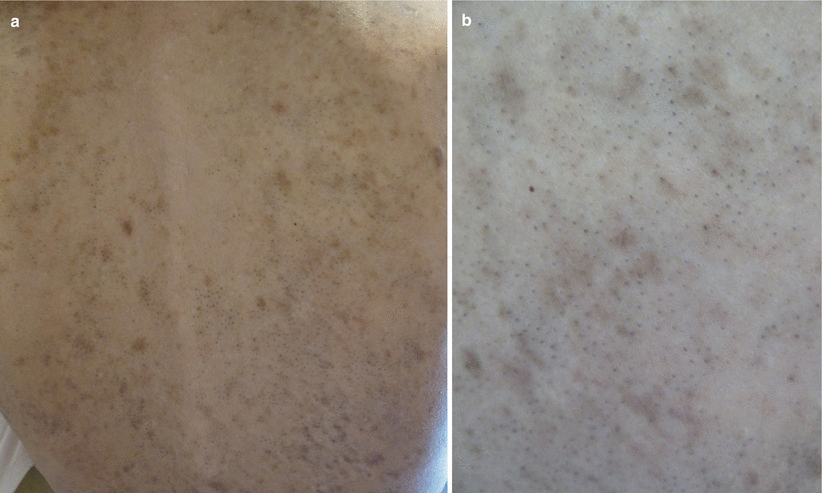

Fig. 12.3
Chronic GVHD manifesting as follicular hyperkeratosis on the flank

Fig. 12.4
(a, b) Chronic GVHD presenting with comedone-like lesions on the back
Atypical Acral Lesions in GVHD
GVHD-Associated Angiomatosis
Eruptive angiomas are an uncommon and poorly understood manifestation of cGVHD [29–33]. The term “GVHD–associated angiomatosis” (GVHD-AA) has been proposed to describe this entity, which is believed to fall within the spectrum of reactive angiomatosis [30]. In GVHD-associated angiomatosis, patients with sclerotic cGVHD develop vascular plaques and nodules within areas of skin fibrosis [30]. Histologically, these lesions display hemorrhagic crust, irregular epidermal acanthosis, or atrophy overlying vague lobular architecture with endothelial proliferation and fibroblast-rich stroma, without atypia [30]. The pathogenesis of these lesions is not clearly understood but may involve increased lymphatic pressure, elevated angiogenic cytokines, and aberrant endothelial damage and repair in the setting of tissue chimerism [30]. These lesions have been associated with a poor prognosis, primarily because of their association with active GVHD and their recalcitrant nature. Data on the management of GVHD-AA are limited, and modalities such as shave, excision, cryotherapy, radiotherapy, thalidomide, and electrocautery have been largely unsuccessful [29–33]. Some success has been demonstrated with combination sirolimus and propranolol [30].
GVHD-Mimicking Connective Tissue Diseases
Chronic cutaneous GVHD may present with an eruption resembling cutaneous lupus erythematosus (LE) [4, 34, 35]. Skin exam may demonstrate a variety of clinical features similar to LE, including a malar rash [4, 35], lesions resembling hypertrophic LE [34], and annular plaques resembling subacute cutaneous lupus erythematosus (SCLE) (Fig. 12.5). Skin biopsy demonstrates features of lichenoid cGVHD, but may also show characteristics seen in LE [34, 35]. This clinical presentation can be associated with a poor prognosis, with either development of sclerotic GVHD or a relapse of hematological disease, as seen in one case series of five patients who presented with malar rash [35]. While auto-antibodies such as antinuclear antibody, anti-Ro, and anti-La are variably positive in chronic GVHD, their significance is unknown. This presentation is not associated with the development of systemic LE symptoms.
Chronic cutaneous GVHD resembling dermatomyositis has been described in a patient who developed a heliotrope rash, edema of the eyelids, erythema of the knuckles, and weakness of proximal muscles with myalgias. Muscle biopsy was characteristic of dermatomyositis, and skin biopsy had features of both GVHD and dermatomyositis [36]. Similarly, chronic cutaneous GVHD can also rarely present with overlapping features of both dermatomyositis and LE [82].


Fig. 12.5
Chronic GVHD mimicking the annular lesions of subacute cutaneous lupus
Unusual Distributions of GVHD
There have been numerous cases of chronic lichen planus-like GVHD presenting along Blaschko’s lines (Fig. 12.6) [37–44]. One hypothesis explaining the linear and whorled distribution of these lesions is an unmasking of genetic mosaicism by the donor’s lymphocytes recognizing altered cell surface antigens which were previously tolerated by the patient’s own lymphocytes [43].
Chronic sclerotic-type and lichen planus-like GVHD may rarely occur in a dermatomal distribution [12, 35, 39, 45–56], often, but not always, at the site of antecedent zoster eruption. It has been hypothesized that viral proteins could play a role by altering the surface antigenicity of keratinocytes, which then serve as targets for the donor effector cells [38, 49, 50, 63, 83]. Interestingly, there has been one reported case of extensive chronic GVHD sparing dermatomes previously affected by herpes zoster, demonstrating a Renbök or inverse Koebner isomorphic phenomenon [84].
Chronic GVHD, typically sclerotic-type, has also demonstrated other isotopic and isomorphic responses, with lesions developing at sites of skin friction (waistband, brassiere) [5, 46, 59, 60], previous central venous catheter placement, with [46] or without [60] cellulitis, repeated needle sticks [46], suction blisters [54], Bacillus Calmette Guerin vaccination [54], influenza vaccination [52], subcutaneous interferon alpha injections [61], healed lesions of aGVHD [47, 54], previous measles exanthem [57], striae distensae [62], external beam radiotherapy [46, 63–65], sun or ultraviolet exposure [66], and other sites of prior trauma or scar (Fig. 12.7). There has been one reported case of sclerotic-type cGVHD presenting as annular plaques in a patient with prior similar annular morphology of his cutaneous lymphoma [58]. Whether this represents a true isotopic response is unclear. These responses are not necessarily limited to chronic GVHD. There has been one reported case of acute GVHD affecting only the lesional skin of a patient with piebaldism [27]. Overall, these isomorphic, isotopic, and isoradiotopic responses may be unified by the concept of the cutaneous immunocompromised district [85]. The cutaneous immunocompromised district, as described by Ruocco et al., is an area of skin where the local effective immunity has been altered, permitting the development of infection, tumor, or a dysimmune reaction, such as GVHD [85]. Though the exact mechanism of GVHD development at these sites remains unknown, this concept may provide some insight into the pathophysiology of cGVHD.










