Atypical (Dysplastic) Melanocytic Nevi: Introduction
|
Common atypical/dysplastic nevi were first recognized as distinct melanocytic neoplasias during the clinical assessment of melanoma-prone families.1 The nomenclature associated with these lesions also includes B–K moles (recognizing the first two families described whose surnames began with B and K),1 familial atypical multiple mole and melanoma syndrome,2 atypical mole syndrome,3 Clark’s nevus,4 atypical moles, and nevus with architectural disorder (with varying degrees of melanocytic atypia).5 The nomenclature has been contentious. The term dysplastic nevus (DN) is frequently used both for clinical and histologic diagnoses,6,7 and it is the term that will be used in this chapter. Although not covered in this chapter, it is also important to note that the terms dysplastic and atypical may also be occasionally used as a modifier for other melanocytic neoplasias (i.e., Spitz nevi) or hyperplasias to indicate a distinct histologic variant from a typical pattern or an increased concern for malignancy.
Epidemiology
DN are frequently found in melanoma-prone families in North America, Europe, and Australia.8–16 One cross-sectional study in a New Zealand cohort (independent of melanoma) revealed DN in 9% of European descent adults.17 A joint case-control study of nevi and melanoma in Australia and the United Kingdom demonstrated that DN were three times more frequent in the Australian controls (6%) than in the British controls (2%).18 There are a few case reports of DN in Japanese, primarily in melanoma-prone families.19
Etiology and Pathogenesis
DN occur in a familial pattern; limited segregation analyses suggest an autosomal dominant transmission of the trait.20 However germ-line susceptibility genes have not yet been identified for DN. Germ-line mutation testing of candidate genes for melanoma (e.g., PTEN, BRAF, and CDK4) did not reveal an association with DN.21 Germ-line polymorphisms in BRAF were also not related to nevi or freckles in another study.22 Within families having CDKN2A germ-line mutations, DN appear to be an independent risk factor for melanoma.11,23 Earlier, linkage analyses in twin studies found evidence of a gene for increased number of nevi in the region around CDKN2A24,25 and recent genome wide association studies suggest that is close to MTAP26,27. Areas of modest interest have also been linked to areas on chromosomes 1, 6, and X.25 Thus, it is likely that DN, like melanoma, is a complex, heterogeneous trait.
Genetic analysis of the cells in DN has revealed activating mutations of BRAF,28,29 or NRAS,30 as well as hemizygous deletions of p16 and p53.31 However no consistent somatic mutation has been noted that defines the DN pattern.
Several lines of evidence suggest that sun or ultraviolet light (UV) exposure is important in the etiology of DN. First, the lesions occur most frequently in sun-exposed areas, particularly in those intermittently exposed, but DN may also occur in unexposed areas.8,32–35 The most common area for DN is on the trunk, primarily the back (Fig. 123-1). There is relative sparing of the more sun-protected lateral thoracic regions and the inner aspect of the arms. Fewer DN occur in doubly covered areas such as on the buttocks. In a comparison of Australian and British controls from a joint case-control study, the prevalence of DN was three times higher overall in the Australians, but the prevalence of nevi on non–sun-exposed areas was the same.18 Second, several studies have also demonstrated, to varying degrees, that individuals with DN report more frequent sun exposure and sunburns, especially before age 20, than individuals without DN.36–38 Individuals who tan well appear to develop fewer DN. Third, based on longitudinal studies of melanoma-prone families, individuals with DN who use sun-protective measures consistently develop fewer new nevi, and the dysplastic and common nevi they have are more likely to involute and disappear.35 Sun-protected children in these high-risk families also develop fewer nevi.
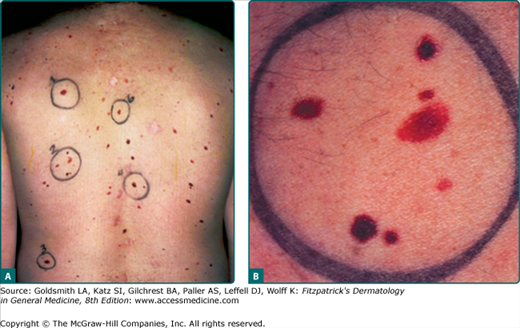
Figure 123-1
A. A back view showing the distribution of dysplastic nevus (DN) and lesion-to-lesion variability, with an increased number of common acquired nevi also. B. A closer view of a cluster of lesions marked as #2 on the back. The lesions are of different sizes, colors, and shapes, with differing amounts of asymmetry, and indistinct borders. Only the largest lesion meets all of the criteria for a clinical DN.
![]() Much of the natural history information about DN derives from longitudinal studies of melanoma-prone families.35 In these families, DN may start to appear early in childhood. The first finding is an increased number of small typical nevi, most often in sun-exposed areas.39 Atypical lesions may also first appear on the scalp of young children,40 possibly due to UV exposure not adequately blocked by the fine hair. The greatest period of DN nevus growth appears to be during young adulthood but growth can still occur late into adulthood.41–43 DN are dynamic lesions. In a study of patients with DN and total body photography (TBP) the greatest amount of new lesions and regressed lesions occurred in the patients under the age of 30.43 The number of new and regressed nevi decreased with age while the number of melanomas increased.
Much of the natural history information about DN derives from longitudinal studies of melanoma-prone families.35 In these families, DN may start to appear early in childhood. The first finding is an increased number of small typical nevi, most often in sun-exposed areas.39 Atypical lesions may also first appear on the scalp of young children,40 possibly due to UV exposure not adequately blocked by the fine hair. The greatest period of DN nevus growth appears to be during young adulthood but growth can still occur late into adulthood.41–43 DN are dynamic lesions. In a study of patients with DN and total body photography (TBP) the greatest amount of new lesions and regressed lesions occurred in the patients under the age of 30.43 The number of new and regressed nevi decreased with age while the number of melanomas increased.
![]() Most DN start as ordinary appearing small, symmetric nevi.35 They continue to enlarge in diameter over varying periods of time. Often, one of the first atypical features is a smudging of the border of the lesion, followed by increasing irregularity of outline, and increasing asymmetry. Color variation may appear early. Some lesions will have growth arrest before reaching a diameter of 5 mm. Often the nevi will remain as relatively flat lesions over extended periods of time. Some will develop a central papule and evolve to a more typical compound or dermal nevus. As this occurs, the flat component generally recedes toward the papular component. Over time, most lesions involute and disappear, leaving no obvious trace.35
Most DN start as ordinary appearing small, symmetric nevi.35 They continue to enlarge in diameter over varying periods of time. Often, one of the first atypical features is a smudging of the border of the lesion, followed by increasing irregularity of outline, and increasing asymmetry. Color variation may appear early. Some lesions will have growth arrest before reaching a diameter of 5 mm. Often the nevi will remain as relatively flat lesions over extended periods of time. Some will develop a central papule and evolve to a more typical compound or dermal nevus. As this occurs, the flat component generally recedes toward the papular component. Over time, most lesions involute and disappear, leaving no obvious trace.35
![]() Nevi and DN tend to have periods of relative stability and periods of increased activity, when several new nevi appear and existing nevi change in color, size, or appearance. The reasons for the increased rate of change are not understood. Anecdotally, the changes can occur after increased sun or UV exposure, or in times of hormonal/growth changes such as puberty and pregnancy.35
Nevi and DN tend to have periods of relative stability and periods of increased activity, when several new nevi appear and existing nevi change in color, size, or appearance. The reasons for the increased rate of change are not understood. Anecdotally, the changes can occur after increased sun or UV exposure, or in times of hormonal/growth changes such as puberty and pregnancy.35
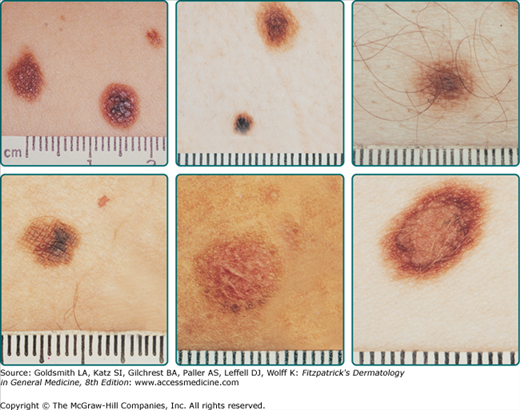
The usual clinical criteria for the diagnosis of a DN are two obligate features: diameter in one dimension at least 5 mm and a prominent flat component, and two of three other features: irregular, asymmetric outline, indistinct borders, and variable pigmentation (Fig. 123-3).44 The histologic features include the presence of an immature or disordered growth pattern with a lymphocytic host response and random cytologic atypia in melanocytes (Fig. 123-4).45 Multiple studies have demonstrated that the clinical and histologic criteria are each reproducible across observers.46–49
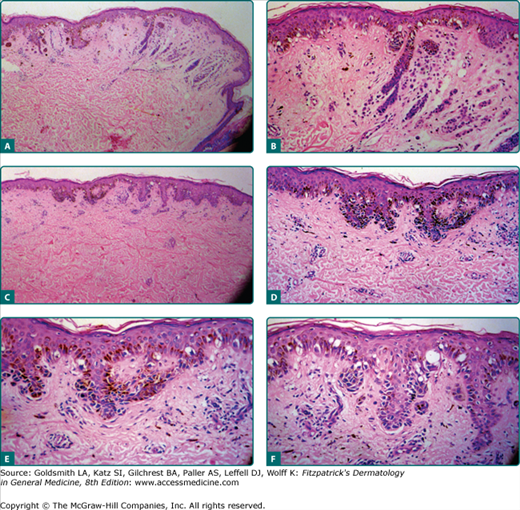
Figure 123-4
The histopathology of a dysplastic nevus (DN). A. The low-power view shows a dermal nevus with dysplasia. B. A closer view of the dysplasia at the shoulder of the dermal nevus. C. Another area of dysplasia. D. On closer view, there is an increased number of melanocytes without an apparent increase in keratinocytes, with the melanocytes clustered on the sides and the ends of the retia. The nuclei of the melanocytes are quite atypical. E. At higher power, the nuclei of the melanocytes are atypical and enlarged, characteristic of epithelioid melanocytic dysplasia. F. Another area of the lesion with melanocytes in linear array, characteristic of lentiginous melanocytic dysplasia. The melanocytes are not as enlarged as in the area of epithelioid dysplasia. With >30 atypical melanocytes and the level of disorganization of the architecture, the lesion is classified as severely dysplastic. (Photomicrographs used with permission from Wallace H. Clark, Jr.)
Typically, DN are most frequent on intermittently exposed skin surfaces.32–35 Epidemiologic studies have demonstrated that the most frequent site is the back (see Fig. 123-1A). DN can, however, occur in minimally sun-exposed areas such as the doubly covered buttocks, breasts, scalp, and pubic regions. Individuals with DN may also develop nevi, not necessarily atypical, on palmar and plantar surfaces and in web spaces of hands and feet. It is uncommon for individuals with DN to have nevi on mucosal surfaces, but they do occur.
Compared to common acquired nevomelanocytic nevi, DN tend to be larger, be entirely flat or have flat shoulders, with a degree of asymmetry and variable coloration (see Fig. 123-3). There may also be a degree of erythema caused by the inflammatory infiltrate in the DN. Within an individual, there is lesion-to-lesion variability, but there tends to be some consistency in the appearance (see Fig. 123-1B). Between individuals, even within the same family, there can be very broad differences in appearance of DN.35 Any specific lesion needs to be assessed in the context of where it is in its natural life history.
Melanoma is the most important related physical finding in patients with DN. The majority of melanomas develop in normal skin, but may also occur in DN, or in common acquired nevi.43,50 The challenge is to identify the melanomas as early as possible (see Chapter 124). Compared to DN, melanomas will be found to be (1) unusual (ugly duckling51), this is due to the rare combination of mutations giving rise to the melanoma and its growth pattern, they will be (2) growing (this due to defective growth control), and progressively more (3) nonuniform (asymmetry, border, color, dermoscopic structures, ABCDs) this is likely due to genomic instability. Nonuniform characteristics may not be present in early in situ melanomas.50
Patients with DN also have elevated numbers of common acquired nevomelanocytic nevi. Other physical findings that may increase melanoma risk in an individual with DN include extent of solar injury and cutaneous phenotype, both of which are independent risk factors for melanoma.52 Evidence of chronic solar injury is also associated with an increased risk of melanoma in men with DN, especially in older men.52 Individuals who are heavily freckled and have DN are at higher risk of developing melanoma than those with DN who are not freckled.44 DN and variations in MC1R both increase the risk of melanoma in CDKN2A mutation carriers.53
The histologic diagnosis of DN can be based on architectural and cytologic features.54 Architectural features include a junctional component of scattered atypical melanocytes which may be most of the lesion, or an edge or shoulder extending several retia beyond a dermal component. Rete ridges are elongated, with the nests of melanocytes at the tips or along the side of the retia with occasional bridging of the nests across retia. There is fibroplasia in the dermis, often concentric eosinophilic fibroplasia or lamellar fibroplasia. Lymphocytes tend to be patchy and perivascular. The cytologic features include atypical melanocytes with abundant cytoplasm along the dermal-epidermal junction that are one-third to one-half larger than the melanocytes in the normal surrounding skin.55 The nuclei may be slightly irregular or folded with hyperchromasia and clumping of chromatin. Some may have prominent nucleoli.
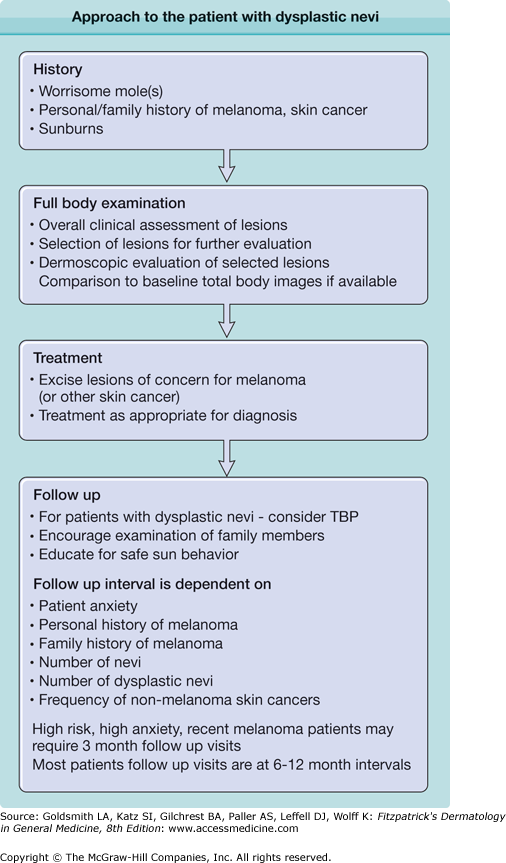
The dermatopathologist should also comment on whether the margins are clear. It is important to communicate with your dermatopathologist on lesions where the clinical and pathologic impressions differ. Marking of worrisome areas on the biopsy or review of dermoscopic images may help insure that particularly troublesome areas have been evaluated to insure that melanoma is not missed. Several groups have proposed criteria for grading histologic dysplasia.48,56–58 These grading systems may help to define the risk of the lesion actually being a melanoma. Lesions with severe atypia may warrant re-excision or closer follow up after complete excision.
Dermoscopy (dermatoscopy or epiluminescence microscopy) is also a useful adjunct for examining lesions. Several sets of criteria for differentiating nevi, including DN from melanoma, have been developed, including pattern analysis,59 ABCD rule,60 Menzie’s method,61 seven-point checklist,62 and color, architecture, symmetry, and homogeneity (CASH).63 The use of dermoscopy has been shown to increase the accuracy of melanoma detection.64
Dermoscopic imaging for follow up may also be considered when a lesion is of concern but not sufficiently worrisome to excise. Often these imaged lesions are then followed and excised only if changes are noted.65,66 In one study with 90-day follow-up identified melanomas, all early, and avoided unnecessary biopsies.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree