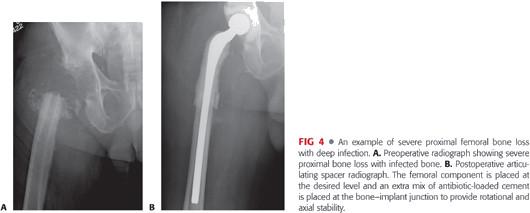
Resection arthroplasty and insertion of a spacer is used for the management of chronic deep periprosthetic infection of the hip.
 This chapter discusses the diagnosis and management of late chronic infections. Acute infections, described in the following text, have a different presentation, methods of diagnosis, and management algorithm.
This chapter discusses the diagnosis and management of late chronic infections. Acute infections, described in the following text, have a different presentation, methods of diagnosis, and management algorithm.
 Antibiotic-loaded spacers are an adjuvant treatment for the management of deep infection by providing elusion of antibiotics into the local tissues.6
Antibiotic-loaded spacers are an adjuvant treatment for the management of deep infection by providing elusion of antibiotics into the local tissues.6
Historically, deep periprosthetic infection was treated by resection arthroplasty alone.
However, depending on the type used, spacers allow for improved function between resection and reimplantation when compared with resection arthroplasty alone by providing soft tissue tension and an articulating surface and, in most cases, allow weight bearing through the lower extremity.
 Spacers can be grouped into articulating spacers or nonarticulating (static) spacers.
Spacers can be grouped into articulating spacers or nonarticulating (static) spacers.
Articulating spacers can resemble either a total hip replacement, with antibiotic-loaded implants inserted on the acetabular and femoral sides, or a hemiarticulating spacer, with an antibiotic-loaded implant inserted only on the femoral side.
Static spacers are blocks and dowels of antibiotic-loaded cement placed into the acetabulum and femoral canal after removal of the implants.
ANATOMY
 The pertinent anatomy of the hip is shown in FIG 1.
The pertinent anatomy of the hip is shown in FIG 1.
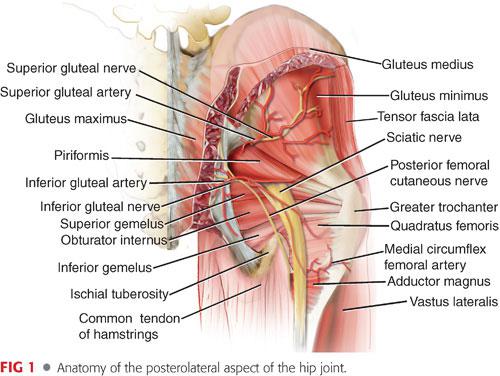
 The fasciae lata cover the musculature of the hip.
The fasciae lata cover the musculature of the hip.
Distally, the fibers condense and form the iliotibial band, which inserts onto the lateral aspect of the proximal tibia (Gerdy tubercle).
Proximally, the fascia splits and envelops the gluteus maximus (inferior gluteal nerve) and the tensor fascia lata (superior gluteal nerve).
 Deep to the fascia lata, over the lateral aspect of the hip, are the major abductors: the gluteus medius and minimus (superior gluteal nerve).
Deep to the fascia lata, over the lateral aspect of the hip, are the major abductors: the gluteus medius and minimus (superior gluteal nerve).
 More posteriorly, deep to the gluteus maximus, are the short external rotators.
More posteriorly, deep to the gluteus maximus, are the short external rotators.
From proximal to distal: piriformis (branches from S1 and S2), superior gemellus (nerve to obturator internus), obturator internus (nerve to obturator internus), inferior gemellus (nerve to quadratus femoris); slightly deeper is the obturator externus (posterior branch obturator nerve); distally is the quadratus femoris (nerve to quadratus femoris).
 The sciatic nerve usually emerges from the lower border of the piriformis and is posterior to the short external rotators.
The sciatic nerve usually emerges from the lower border of the piriformis and is posterior to the short external rotators.
When approaching the hip posteriorly, retracting the short rotators posteriorly will provide some protection to the sciatic nerve.
 An ascending branch from the medial femoral circumflex courses over the posterior aspect of the quadratus femoris; this may produce bleeding during dissection.
An ascending branch from the medial femoral circumflex courses over the posterior aspect of the quadratus femoris; this may produce bleeding during dissection.
 Anterior to the hip capsule is the iliopsoas tendon on which the femoral nerve lies as it crosses under the ilioinguinal ligament and enters the thigh.
Anterior to the hip capsule is the iliopsoas tendon on which the femoral nerve lies as it crosses under the ilioinguinal ligament and enters the thigh.
Retractors placed over the anterior wall need to be placed directly on bone to avoid injury to the nerve.
PATHOGENESIS
 Periprosthetic infections are classified as acute or chronic infections (Table 1).9,30
Periprosthetic infections are classified as acute or chronic infections (Table 1).9,30
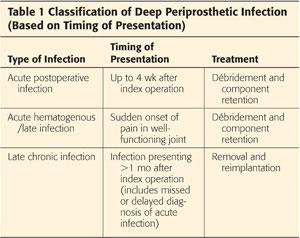
 Acute infections are either acute postoperative infections or acute hematogenous (late) infections.
Acute infections are either acute postoperative infections or acute hematogenous (late) infections.
If diagnosed early, acute infections can be managed with débridement and irrigation with component retention.
 Chronic infections generally present in a delayed manner, usually months or occasionally years after the index procedure. The infection has likely been present since the original procedure, but because of the low virulence of the infecting organism, classic signs of infection are lacking, and hip pain may be the only presenting symptoms.
Chronic infections generally present in a delayed manner, usually months or occasionally years after the index procedure. The infection has likely been present since the original procedure, but because of the low virulence of the infecting organism, classic signs of infection are lacking, and hip pain may be the only presenting symptoms.
 Chronic infections also include a missed or delayed diagnosis of an acute infection. A missed or delayed diagnosis of an acute infection must now be treated as a chronic infection.
Chronic infections also include a missed or delayed diagnosis of an acute infection. A missed or delayed diagnosis of an acute infection must now be treated as a chronic infection.
NATURAL HISTORY
 Chronic periprosthetic infection will continue to cause pain and disability.
Chronic periprosthetic infection will continue to cause pain and disability.
 The severity of symptoms depends on the virulence of the organism, the overall health or comorbidities of the patient, and fixation of the implants and status of the surrounding soft tissues.
The severity of symptoms depends on the virulence of the organism, the overall health or comorbidities of the patient, and fixation of the implants and status of the surrounding soft tissues.
 Low-virulence organisms (eg, coagulase-negative Staphylococcus) may present with chronic pain, whereas more virulent organisms (eg, Staphylococcus aureus) or hosts that are immunocompromised may present with more obvious signs of infection.
Low-virulence organisms (eg, coagulase-negative Staphylococcus) may present with chronic pain, whereas more virulent organisms (eg, Staphylococcus aureus) or hosts that are immunocompromised may present with more obvious signs of infection.
 Untreated patients are at risk for seeding other joint replacements. The incidence is unknown and likely depends on the virulence of the organism and the host’s medical comorbidities.
Untreated patients are at risk for seeding other joint replacements. The incidence is unknown and likely depends on the virulence of the organism and the host’s medical comorbidities.
Patients who have multiple medical comorbidities or are infected with more virulent organisms may be at greater risk for signs or symptoms of systemic infection and, subsequently, seeding of other joints by hematogenous spread.
 With time, chronic infection can cause bone loss and loosening of implants.
With time, chronic infection can cause bone loss and loosening of implants.
In addition to increased pain from implant loosening, the osteolysis, which may be secondary to both infection and loose implants, can result in an increased risk of periprosthetic fracture.
PATIENT HISTORY AND PHYSICAL FINDINGS
 In most cases, a careful history will lead to suspicion of a diagnosis of chronic infection.
In most cases, a careful history will lead to suspicion of a diagnosis of chronic infection.
 Often, patients give a history of poor wound healing, with prolonged drainage, prolonged antibiotic use, or surgical débridement.
Often, patients give a history of poor wound healing, with prolonged drainage, prolonged antibiotic use, or surgical débridement.
These cases represent missed or failed treatment for acute postoperative infections. Missed acute hematogenous (late) infections, which are now chronic, present with a history of sudden deterioration in hip function, without other obvious mechanical causes of failure.
 Another subset of patients with chronic infection present only with pain. Often, it has been present since the time of the initial index procedure.
Another subset of patients with chronic infection present only with pain. Often, it has been present since the time of the initial index procedure.
There may also be a history of poor wound healing or prolonged drainage.
The pain is usually different in character than preoperative activity-related arthritic pain. It may be more constant, present at rest, and consist of a dull ache. The pain may worsen with activity but tends to be more constant than pure mechanically related pain from a loose implant.
 The physical examination is often nonspecific in the setting of chronic infection.
The physical examination is often nonspecific in the setting of chronic infection.
The examination findings range from nearly normal, with only mild pain with range of motion, to more obvious signs of infection, such as a chronically draining sinus.
 Examinations to perform include the following:
Examinations to perform include the following:
Observe gait pattern. Pain or muscle weakness may cause limp. Trunk may shift over affected hip.
Trendelenburg sign. A positive result may indicate abductor dysfunction, pain, or a neurologic problem (superior gluteal nerve or L5 nerve root).
Inspect any old incision sites and surrounding skin. Plan to incorporate all or as much of the old incisions as possible. A draining sinus indicates a deep infection.
Examine the soft tissue for thickness and compliance. Poor tissue compliance may compromise closure and wound healing. Poor tissue integrity may require rotation flap for closure.
Passive range of motion. Significant stiffness may make surgical exposure more difficult. Excessive motion may increase the risk of instability.
Straight-leg raising may be limited by pain from infection, loose implants, or hip flexor tendonitis.
Assess true and apparent leg lengths.
Perform a neurovascular examination. Check and document the status of motor group function, sensation, and pulses preoperatively in case of any change following surgery.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Radiographs
Radiographs
In most cases, radiographs do not show signs of chronic infection. Radiographs are necessary to exclude other causes of aseptic failure and for surgical planning.
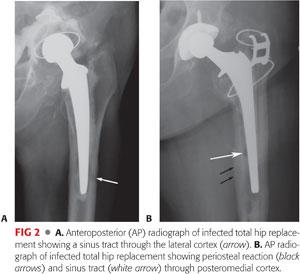
Radiographs from patients with long-standing chronic infection will show signs of deep infection. A periosteal reaction is considered pathognomonic for deep infection. Sinus tracks, extending through bone, may rarely be seen (FIG 2).
 Laboratory investigations
Laboratory investigations
The most useful laboratory tests, both to confirm and to exclude a suspected diagnosis of deep infection, are the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
• Various values have been considered positive (indicative of infection). It is generally accepted that an ESR above 30 mm per hour and a CRP above 10 mg/L are indicators of potential infection, provided the patient does not have other diagnoses that lead to elevated inflammatory markers.27
• The sensitivity of the ESR or CRP, used individually, is diminished in the setting of nonvirulent chronic indolent infections.22 However, when used in combination, if both values are strongly negative (low normal), it is unlikely that the patient is infected, and other diagnoses should be considered.
White blood cell count is rarely abnormal in chronic infection and is not helpful for diagnosing deep infection.
Hip aspiration for culture and synovial white blood cell count is indicated if there is any clinical suspicion of infection or if there is elevation of either the ESR or CRP. Any antibiotics that the patient may be receiving should be discontinued at least 2 to 3 weeks before aspiration to reduce the risk of false-negative cultures.2
• The specimen should be separated into two, or preferably three, specimens for culture. If all cultures are positive for the same organism and the results correlate with the clinical presentation and elevation of inflammatory markers, then the diagnosis is confirmed.
• The aspiration is routinely sent for aerobic and anaerobic cultures. In patients who have been previously investigated or managed for presumed infection with negative cultures or in patients who are immunosuppressed (eg, transplant patients, HIV-positive patients, or cancer patients undergoing chemotherapy), the specimen is also sent for fungal and mycobacterial cultures.
Synovial white blood cell count has become a useful method to help diagnose deep infection. Synovial cell count values diagnosing infection are mostly based on knee aspiration results, with only one study reporting data from hip aspirations. Although results vary, generally, a synovial white blood cell count of greater than 3000 cells/μL or over 70% polymorphonuclear leukocytes is suggestive of infection.15,23,29
 Frozen section is a helpful intraoperative test, but as with other investigations, it must be interpreted within the context of the clinical presentation and the results of other investigations; no single test is 100% reliable. The sensitivity and specificity of frozen section are approximately 0.80 and 0.90, respectively.25
Frozen section is a helpful intraoperative test, but as with other investigations, it must be interpreted within the context of the clinical presentation and the results of other investigations; no single test is 100% reliable. The sensitivity and specificity of frozen section are approximately 0.80 and 0.90, respectively.25
Tissue for frozen section should be obtained from the areas that look most inflamed. A positive result, indicative of infection, is considered when there are more than five polymorphonuclear leukocytes per high-power field.18
 Intraoperative Gram stain should not be used to determine the presence or absence of infection. In late chronic infection, it has an extremely poor sensitivity.26
Intraoperative Gram stain should not be used to determine the presence or absence of infection. In late chronic infection, it has an extremely poor sensitivity.26
 In 2010, the American Academy of Orthopaedic Surgeons1 (AAOS) published the first set of Clinical Practice Guidelines on the Diagnosis of Periprosthetic Joint Infections of the Hip and Knee. The diagnostic workup algorithms are based on the pretest probability of infection. The pretest probability of infection (either high or low) is based on the clinical presentation of the patient. The guideline recommendations and investigation algorithms are available on the AAOS website.1
In 2010, the American Academy of Orthopaedic Surgeons1 (AAOS) published the first set of Clinical Practice Guidelines on the Diagnosis of Periprosthetic Joint Infections of the Hip and Knee. The diagnostic workup algorithms are based on the pretest probability of infection. The pretest probability of infection (either high or low) is based on the clinical presentation of the patient. The guideline recommendations and investigation algorithms are available on the AAOS website.1
 The diagnosis of chronic periprosthetic infection is made by the interpretation of clinical findings and the investigations mentioned earlier. The Musculoskeletal Infection Society established criteria for the diagnosis of periprosthetic joint infection.20 Recently, these criteria were updated at the International Consensus Meeting on Periprosthetic Joint Infection.33 The updated definition of periprosthetic joint infection is outlined in Table 2.
The diagnosis of chronic periprosthetic infection is made by the interpretation of clinical findings and the investigations mentioned earlier. The Musculoskeletal Infection Society established criteria for the diagnosis of periprosthetic joint infection.20 Recently, these criteria were updated at the International Consensus Meeting on Periprosthetic Joint Infection.33 The updated definition of periprosthetic joint infection is outlined in Table 2.

DIFFERENTIAL DIAGNOSIS
 Intrinsic
Intrinsic
Aseptic loosening
Fibrous ingrowth (uncemented implants)
Polyethylene wear with synovitis
Modulus mismatch
Tendinitis (eg, psoas tendon impingement)
Bursitis or degenerative abductor avulsion
Heterotopic ossification
Stress fracture
 Extrinsic
Extrinsic
Spinal pathology (eg, L2 nerve root impingement)
Vascular claudication
Hernia
Lateral femoral cutaneous nerve impingement
NONOPERATIVE MANAGEMENT
 In established cases of chronic infection, nonoperative management is rarely indicated for definitive treatment.
In established cases of chronic infection, nonoperative management is rarely indicated for definitive treatment.
 Once the organism has been identified, antibiotic suppressive therapy may be used as a temporizing measure.
Once the organism has been identified, antibiotic suppressive therapy may be used as a temporizing measure.
Antibiotic treatment may be able to suppress the infection and will likely prevent bacteremia if surgical treatment has to be delayed.
Antibiotic suppression may be considered in patients with very limited life expectancy who have a reasonably well-functioning joint, provided that the infecting organism has been identified and the infection can be suppressed with a well-tolerated oral antibiotic.
The use of antibiotics alone will not eradicate an established chronic infection, and antibiotic therapy alone should not be used if curing the infection is the goal.
SURGICAL MANAGEMENT
 The favored method of management of chronic periprosthetic hip infection is a two-stage exchange: removal of all implants and foreign material, followed by delayed reimplantation.
The favored method of management of chronic periprosthetic hip infection is a two-stage exchange: removal of all implants and foreign material, followed by delayed reimplantation.
The time between stages allows the surgeon to observe the patient’s response to therapy, thereby allowing him or her to assess for the possibility of recurrence of infection after the antibiotics have been stopped and before reimplantation.
 The principles of surgical management during the first stage are removal of the implants and all foreign material, thorough débridement of the joint, and insertion of a high-dose antibiotic cement spacer (either articulating or static).
The principles of surgical management during the first stage are removal of the implants and all foreign material, thorough débridement of the joint, and insertion of a high-dose antibiotic cement spacer (either articulating or static).
 The patient is then treated with the appropriate antibiotic therapy, in addition to medical and nutritional management. This is ideally followed by a period of time off all antibiotics to ensure clinical resolution of infection, after which the second-stage reimplantation is performed.
The patient is then treated with the appropriate antibiotic therapy, in addition to medical and nutritional management. This is ideally followed by a period of time off all antibiotics to ensure clinical resolution of infection, after which the second-stage reimplantation is performed.
 The principles of reconstruction during the second stage are as for aseptic revisions and are independent of the infection.
The principles of reconstruction during the second stage are as for aseptic revisions and are independent of the infection.
If infection is suspected at the time of reimplantation, definitive reconstruction should not be performed and the patient should instead be treated with repeat débridement and insertion of a spacer.
Otherwise, if the pre-reimplantation workup is negative and if there is no suspicion of infection at the time of reimplantation, it is assumed that the patient is free of infection, and reconstruction should be performed in a manner that is likely to give stable fixation of the implants and the best chance for good long-term functional outcome.
 In most cases, an uncemented reconstruction is favored.7,8,17,19,28 The use of cement is not specifically indicated for the second stage of reconstruction when a two-stage approach is used.
In most cases, an uncemented reconstruction is favored.7,8,17,19,28 The use of cement is not specifically indicated for the second stage of reconstruction when a two-stage approach is used.
 A variety of techniques have been described to create spacers after removal of the implants.3,7,8,11,16,24 The common practice involves using high-dose antibiotics in the bone cement to obtain high local antibiotic concentrations.
A variety of techniques have been described to create spacers after removal of the implants.3,7,8,11,16,24 The common practice involves using high-dose antibiotics in the bone cement to obtain high local antibiotic concentrations.
Prefabricated commercially available spacers currently contain only low doses (generally considered prophylactic levels) of antibiotics. When used, these spacers are often inserted with the addition of high-dose antibiotic-loaded cement. Currently, we recommend against the use of these spacers and favor making spacers intraoperatively with high-dose antibiotics tailored to the organism.
We use the prosthesis with antibiotic-loaded acrylic cement (Prostalac [Depuy Synthes, Warsaw, Indiana]) molds,5 which are described later. The technique can be adapted to other mold systems, however.
Preoperative Planning
 Preoperative planning is similar to any revision hip replacement procedure. Planning the steps used for managing the infection and inserting an antibiotic spacer is also required.
Preoperative planning is similar to any revision hip replacement procedure. Planning the steps used for managing the infection and inserting an antibiotic spacer is also required.
These steps include ensuring that the patient is medically stable to undergo the procedure, having the appropriate equipment available to remove the implants (eg, high-speed burrs, thin blade saws, ultrasonic cement removal equipment, trephines, or acetabular removal systems), and having equipment on hand for making the antibiotic-loaded spacer intraoperatively.
 Management of a chronically infected total hip replacement requires that every reasonable attempt be made to identify the organism preoperatively.
Management of a chronically infected total hip replacement requires that every reasonable attempt be made to identify the organism preoperatively.
In most cases, the antibiotics used in the bone cement will be the same, although occasionally, an atypical organism will be identified preoperatively, requiring alteration in the content of antibiotics mixed into the bone cement.
 It is unlikely that nonimmunosuppressed patients with chronic infections will become bacteremic if antibiotics are stopped for a short period of time. Therefore, antibiotics that the patient may be receiving preoperatively should be discontinued about 2 to 4 weeks before surgery to improve the yield of positive cultures at the time of surgery.
It is unlikely that nonimmunosuppressed patients with chronic infections will become bacteremic if antibiotics are stopped for a short period of time. Therefore, antibiotics that the patient may be receiving preoperatively should be discontinued about 2 to 4 weeks before surgery to improve the yield of positive cultures at the time of surgery.
Occasionally, a second organism or an organism with a different antibiotic sensitivity profile than that obtained preoperatively will be identified from intraoperative cultures.
 The appropriate molds and antibiotics to be mixed into the cement need to be available to make the spacer intraoperatively.
The appropriate molds and antibiotics to be mixed into the cement need to be available to make the spacer intraoperatively.
The most commonly used antibiotics are vancomycin and gentamicin or tobramycin. Other antibiotics can be used as well (Tables 3 and 4).
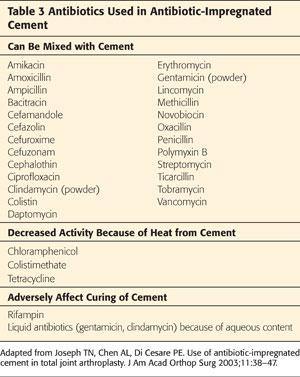
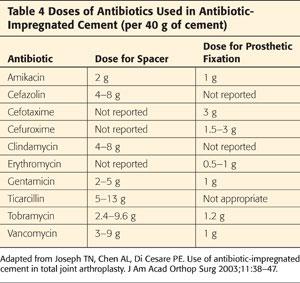
 Intrapelvic cement, if present, needs to be identified preoperatively.
Intrapelvic cement, if present, needs to be identified preoperatively.
Small amounts can be removed from the defect within the floor of the acetabulum.
Large amounts of cement require a preoperative contrast computed tomography (CT) scan to assess the location of cement relative to intrapelvic structures. A separate retroperitoneal approach may be required and should be planned.
 Radiolucent bone cement also requires CT evaluation preoperatively to determine its distal extent within the femoral canal or within the pelvis.
Radiolucent bone cement also requires CT evaluation preoperatively to determine its distal extent within the femoral canal or within the pelvis.
Positioning
 Patients are positioned as for other revision hip procedures.
Patients are positioned as for other revision hip procedures.
 The lateral decubitus position, with the affected hip upward, is favored. The extremity is freedraped with sufficient exposure of skin to allow for extension of the incision as required.
The lateral decubitus position, with the affected hip upward, is favored. The extremity is freedraped with sufficient exposure of skin to allow for extension of the incision as required.
 If a retroperitoneal approach is required for removal of medial cement, the patient is positioned supine for this portion of the procedure.
If a retroperitoneal approach is required for removal of medial cement, the patient is positioned supine for this portion of the procedure.
Approach
 The approach depends on the fixation status of the implants, length of the cement column if present, quality of bone, and stiffness of the hip joint.
The approach depends on the fixation status of the implants, length of the cement column if present, quality of bone, and stiffness of the hip joint.
 The primary goals of the exposure are to allow for safe, efficient, and thorough removal of the implants and cement or other foreign material and to allow for thorough débridement of the joint.
The primary goals of the exposure are to allow for safe, efficient, and thorough removal of the implants and cement or other foreign material and to allow for thorough débridement of the joint.
 An extended trochanteric osteotomy is usually used. This provides for excellent exposure of the acetabulum and allows for safe removal of the femoral component.
An extended trochanteric osteotomy is usually used. This provides for excellent exposure of the acetabulum and allows for safe removal of the femoral component.
 If the femoral component is loose or has minimal proximal fixation, a standard approach can be used. Radiographs must be carefully inspected to ensure that removal of the femoral component can be accomplished from above.
If the femoral component is loose or has minimal proximal fixation, a standard approach can be used. Radiographs must be carefully inspected to ensure that removal of the femoral component can be accomplished from above.
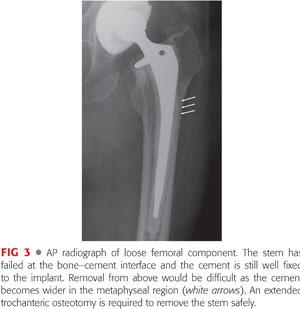
 Varus remodeling of the proximal femur, or cement that is wider distally than proximally and still well fixed to the implant within the canal, impedes removal of the femoral component and increases the risk of fracture (FIG 3).
Varus remodeling of the proximal femur, or cement that is wider distally than proximally and still well fixed to the implant within the canal, impedes removal of the femoral component and increases the risk of fracture (FIG 3).
A posterior approach is favored as it is more extensile and allows for an osteotomy if removal of implants becomes more difficult than expected.
 The skin incision should be made through the previous incision, unless the old incision significantly compromises the exposure of deep structures. A portion of the old incision can usually be incorporated; however, when joining the old incision, avoid acute angles to reduce the risk of wound edge necrosis.
The skin incision should be made through the previous incision, unless the old incision significantly compromises the exposure of deep structures. A portion of the old incision can usually be incorporated; however, when joining the old incision, avoid acute angles to reduce the risk of wound edge necrosis.
The old incision can be excised to give fresh, nonscarred skin edges that may improve wound healing.
 Sinus tracts should be excised elliptically.
Sinus tracts should be excised elliptically.
 Once the joint has been exposed, at least three samples are obtained for culture.
Once the joint has been exposed, at least three samples are obtained for culture.
 Removal of the implants is then carried out as per the techniques of nonseptic revision cases and will not be detailed here (please see corresponding chapters).
Removal of the implants is then carried out as per the techniques of nonseptic revision cases and will not be detailed here (please see corresponding chapters).
Removal of implants and débridement is more thorough than in aseptic revisions and every attempt should be made to remove all foreign and potentially infected material.
TECHNIQUES
 Articulating Antibiotic Spacer
Articulating Antibiotic Spacer
After exposure and removal of the implants, the joint is thoroughly débrided. It is important to remove all foreign material, which is potentially infected.
Retention of cement or other foreign material is associated with an increased failure rate for curing the infection.
Intraoperative radiographs can be used to look for retained cement. An arthroscope passed down the femoral canal can also be used to inspect the canal for remaining cement.
The steps to using a system of molds to make an articulating spacer and to maximize efficiency are as follows:
Remove infected femoral component (and cement if applicable).
Size and make the antibiotic-loaded femoral implant in the appropriate mold.
While the cement for the femoral component is hardening in the mold, remove and débride the acetabulum.
Cement the acetabular component into the acetabulum with antibiotic-loaded cement.
Remove the femoral component from the mold and insert into the femoral canal.
Perform trial reduction.
If the femoral component is not stable within the femoral canal and if there is obvious rotation or risk of significant subsidence, a third batch of antibiotic-loaded cement is mixed. When doughy, the cement is placed around the proximal portion of the femoral spacer as it is reinserted into the femoral canal to the desired level to provide rotational and axial stability to the implant. Ensure that not too much cement stays laterally, which may impede closure of the osteotomy if used.
Reduce the hip and close.
Spacer Creation
For most infections, including methicillin-resistant organisms, the antibiotic mixture used in the cement is 3.6 g of gentamicin or tobramycin, 3 g of vancomycin, and 2 g of cefazolin per pack (40 g) of cement. Palacos is favored, as most studies have indicated superior elution compared with other cements.21 The use of Cefazolin is optional. It is added as a porogen to increase elusion of other antibiotics. It may also provide additional efficacy for methicillin-sensitive organisms.
Most cases require a total of two mixes of cement (one for the acetabulum and one for the femoral mold). If more than two batches are required and the patient is renally impaired (serum creatinine above 1.5 mg/dL), the dose is decreased to 2.4 g of gentamicin (or tobramycin), 2 g of vancomycin, and 2 g of cefazolin per batch of cement.
The Prostalac molds are available in variety of sizes and lengths (120, 150, 200, and 240 mm). The length of implant used depends on the length of the extended trochanteric osteotomy (if used), the amount of bone loss, and the size of the canal.
In most cases, a middle (200 mm) or long (240 mm) stem is chosen as a longer length also helps achieve better rotational and axial stability within the canal.
Antibiotics are mixed with one batch of cement in a mixing bowl and then placed into the mold.
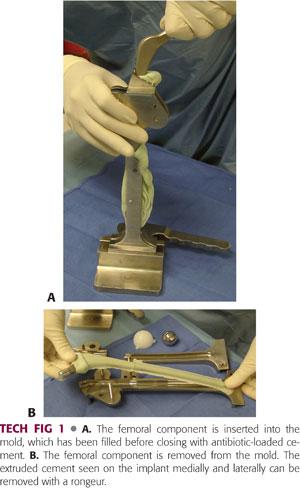
The mold is closed but not fully tightened (to allow for cement to extrude) and the stem is inserted. The mold is then fully tightened and extruded cement is removed from its outer aspect (TECH FIG 1).
Alternatively, after filling the open mold with cement, the implant is laid into the mold, which is closed over the implant.
Implant Placement
While the cement is hardening in the femoral mold, the infected acetabular component is removed and the socket débrided. Acetabular reamers can be used to help with débridement, but excessive bone loss from reaming should be avoided.
A second mix of antibiotic-loaded cement is prepared, and when in a doughy phase, the polyethylene acetabular implant is cemented into the socket.
A “perfect cement technique” should be avoided because this can make removal at the time of reimplantation more difficult.
Waiting until the cement is doughy and applying some but not excessive force when cementing in the liner will provide a stable cup that can be readily removed.
If there are large acetabular defects, leftover cement from the femoral mold can be shaped over a reamer of approximate size to make an “antiprotrusio-like” cup that can be placed into the floor of the acetabulum before cementing in the polyethylene cup (TECH FIG 2A).
Warm saline can be poured into the acetabulum to decrease the setting time.
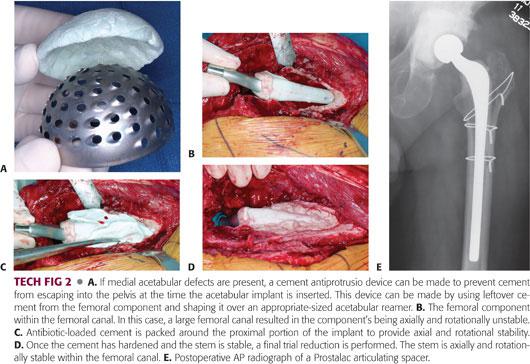
Once the acetabular cement has hardened, the femoral component is removed from the mold (see TECH FIG 1B) and inserted into the femoral canal.
In many cases, the stem is press-fitted into the canal, good rotational and axial stability is obtained, and no further adjustment is required.
In some cases, the fit may be too tight and the antibiotic-loaded implant will not fully seat to the desired level.
A high-speed burr is used to remove high points or areas of impingement to allow the stem to seat at the desired level. Alternatively, flexible reamers can be used to increase the opening of the femoral canal, provided there is ample bone stock.
In other cases, especially with significant bone loss or very large canals, the femoral component is loose within the femoral canal.
A trial reduction is performed to estimate leg lengths, and the desired position of the stem within the femoral canal is noted.
An additional batch of antibiotic-loaded cement is mixed, and when of a doughy consistency, cement is packed around the proximal portion of the femoral spacer and the implant is reinserted to the desired level. The additional cement provides rotational and axial stability (TECH FIG 2B–D).
Once the stem is stable within the femoral canal, a trial reduction can be performed to assess leg lengths and stability.
The appropriate femoral head is then placed onto the stem and the hip is reduced and closed.
The Prostalac articulating antibiotic spacer uses a snap-fit all-polyethylene acetabular implant into which the femoral head is snap-fitted into the cup when reduced, enhancing hip stability (TECH FIG 2E).
The snap-fit poly liner may reduce the risk of dislocation, particularly in situations of proximal femoral bony or soft tissue deficiency.
 Nonarticulating Antibiotic Spacer Block
Nonarticulating Antibiotic Spacer Block
The initial steps for using a nonarticulating spacer are as for the articulating spacer.
This involves ensuring that all the foreign material is removed and the hip is thoroughly débrided.
The same antibiotic concentrations are used in the bone cement.
For an unusual organism, the antibiotics can be adjusted to be organism-specific, but for most infections, the mixture is outlined earlier.
Two mixes of antibiotic-containing bone cement are required: one for the acetabulum and one for the femur.
Once the hip is débrided, one mix of antibiotic-loaded cement of a partially polymerized doughy consistency is placed into the socket. It is molded into the acetabulum, matching the bony contour to give some stability to the cement block to prevent it from migrating or dislodging spontaneously.
The second mix is made into a tapered dowel. Once hardened, the dowel is placed down the femoral canal.
It is important to make a taper so that the dowel can be easily extracted at the time of reimplantation.
The nozzle from a cement gun can be used to make a long tapered dowel (TECH FIG 3).
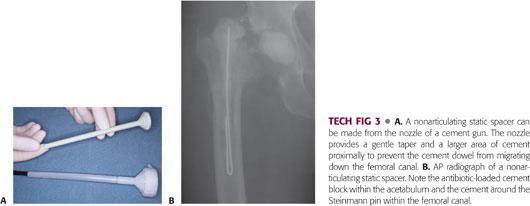
An alternative method is to wrap cement around a threaded pin, again making sure that a taper is created with cement larger proximally to keep the dowel from migrating down the canal.
Once the spacers are inserted, the hip is closed.
PEARLS AND PITFALLS | |
Cementing polyethylene liner for articulating spacer |
|
| |
Large medial acetabular defect |
|
Femoral stem that is rotationally unstable |
|
Severe proximal femoral bone loss |
|
Femoral dowel for nonarticulating spacer |
|
Mixing antibiotics into cement |
|
| |
| |
| |
| |
POSTOPERATIVE CARE
Management of the Infection
 Infection management consists of medical and nutritional support and appropriate antibiotics to treat the infecting organism(s).
Infection management consists of medical and nutritional support and appropriate antibiotics to treat the infecting organism(s).
 The optimal duration and regimen of antibiotics remain controversial.
The optimal duration and regimen of antibiotics remain controversial.
Most authors recommend 6 weeks of intravenous antibiotic therapy, although there is considerable variability in published reports, ranging from 0 to 9 weeks of intravenous antibiotics and none to more than 2 years of oral antibiotics.14
The choice of antibiotics depends on the organism, but there is a tendency (such as with methicillin-resistant staphylococcal infections) to use multiple antibiotics for synergistic effect (eg, vancomycin and rifampin).12
 We favor 6 weeks of antibiotics, at which time the antibiotics are discontinued. Inflammatory markers (ESR and CRP) are repeated at 6 weeks (at the time the antibiotics are stopped). The inflammatory markers often return to normal or trend markedly downward, and reimplantation is planned for around 3 months from insertion of the spacer.
We favor 6 weeks of antibiotics, at which time the antibiotics are discontinued. Inflammatory markers (ESR and CRP) are repeated at 6 weeks (at the time the antibiotics are stopped). The inflammatory markers often return to normal or trend markedly downward, and reimplantation is planned for around 3 months from insertion of the spacer.
 If the ESR and CRP remain elevated at 6 weeks, we favor stopping the antibiotics and following the patient’s course clinically.
If the ESR and CRP remain elevated at 6 weeks, we favor stopping the antibiotics and following the patient’s course clinically.
The ESR and CRP are repeated at 4-week intervals. If they return to normal or have markedly decreased from very high levels preoperatively to near normal, and there are no clinical signs of ongoing infection, then reimplantation can be planned for 3 to 4 months after insertion of the spacer.
 If the inflammatory markers remain elevated at 3 months, the options are as follows:
If the inflammatory markers remain elevated at 3 months, the options are as follows:
Continue to follow the patient clinically, particularly if he or she is functioning well with an articulating spacer, and reinvestigate prior to the planned reimplantation with repeat ESR and CRP as well as a hip aspiration for cell count.
Repeat the débridement and insertion of a new antibiotic spacer.
 Multiple débridements at short intervals, based solely on elevated inflammatory markers, should be avoided.
Multiple débridements at short intervals, based solely on elevated inflammatory markers, should be avoided.
 Routine reaspiration for culture is of limited value as periprosthetic antibiotic levels are often still above minimum inhibitory concentrations at 3 months. Synovial white blood cell count differential may be of greater value to determine the presence or absence of infection prior to reimplantation, although data supporting routine use of this are limited.24
Routine reaspiration for culture is of limited value as periprosthetic antibiotic levels are often still above minimum inhibitory concentrations at 3 months. Synovial white blood cell count differential may be of greater value to determine the presence or absence of infection prior to reimplantation, although data supporting routine use of this are limited.24
Management of the Hip (Spacer)
 Postoperative weight bearing and mobility depend on the type of spacer used.
Postoperative weight bearing and mobility depend on the type of spacer used.
 Most patients with an articulating spacer are very functional between stages, often having minimal pain and ultimately ambulating near full weight bearing with a cane or walker prior to reimplantation.
Most patients with an articulating spacer are very functional between stages, often having minimal pain and ultimately ambulating near full weight bearing with a cane or walker prior to reimplantation.
 Patients with articulating antibiotic spacers that have a stable press-fit with good rotational stability are allowed to mobilize with partial weight bearing (50%) for 6 weeks, followed by weight bearing as tolerated, particularly if follow-up radiographs at 6 weeks show no significant change in implant position.
Patients with articulating antibiotic spacers that have a stable press-fit with good rotational stability are allowed to mobilize with partial weight bearing (50%) for 6 weeks, followed by weight bearing as tolerated, particularly if follow-up radiographs at 6 weeks show no significant change in implant position.
 If there is concern about the stability of the femoral component within the femoral canal (eg, large canal with difficulties getting good rotational or axial stability of the implant in the canal), then the patient is maintained at 50% weight bearing until the time of reimplantation.
If there is concern about the stability of the femoral component within the femoral canal (eg, large canal with difficulties getting good rotational or axial stability of the implant in the canal), then the patient is maintained at 50% weight bearing until the time of reimplantation.
 If a nonarticulating spacer is used, then patients generally cannot bear weight through the lower extremity and are kept touch weight bearing until reimplantation.
If a nonarticulating spacer is used, then patients generally cannot bear weight through the lower extremity and are kept touch weight bearing until reimplantation.
OUTCOMES
 The overall success for curing periprosthetic hip infection using a two-stage exchange technique is approximately 89% to 93%.3,4,7,8,10,14,17,31,32
The overall success for curing periprosthetic hip infection using a two-stage exchange technique is approximately 89% to 93%.3,4,7,8,10,14,17,31,32
 Numerous variables influence the success of treatment:
Numerous variables influence the success of treatment:
Depth of infection
Time from index operation
Prosthetic status (fixation and position)
Soft tissue status
Host status (medical comorbidities)
Pathogen (virulence)
Surgeon capabilities
Patient expectations
 Without the use of antibiotic-loaded cement spacers and without antibiotic-loaded cement at the time of reimplantation, the cure rate for infection using two-stage (delayed) reconstruction is approximately 82%.10
Without the use of antibiotic-loaded cement spacers and without antibiotic-loaded cement at the time of reimplantation, the cure rate for infection using two-stage (delayed) reconstruction is approximately 82%.10
This cure rate is also similar to a one-stage (direct) exchange in which antibiotic-loaded cement is used at the time of the direct exchange. This implies that delayed reconstruction and the use of antibiotic-loaded spacers are in part responsible for the improved success rate when treating infected total hip replacements.
 Combining a series of patients treated with a two-stage (delayed) reconstruction without antibiotic spacers but with antibiotic-loaded cement at the time of reimplantation reveals a success rate of approximately 90%.10
Combining a series of patients treated with a two-stage (delayed) reconstruction without antibiotic spacers but with antibiotic-loaded cement at the time of reimplantation reveals a success rate of approximately 90%.10
Patients treated with a two-stage (delayed) reconstruction using antibiotic-loaded spacers and uncemented reconstruction show a similar success rate to those treated with spacers and antibiotic-loaded cement at the time of reconstruction. This success rate is approximately 89% to 93%.3,4,10,31,32
Uncemented reconstruction at the time of reimplantation, when used with antibiotic-loaded spacers, has not resulted in a lower infection cure rate.7,8,17 Also, uncemented reconstruction will likely result in a better long-term mechanical survival.
 Using the articulating Prostalac antibiotic-loaded spacer has an infection cure rate of 93% (45/48 patients).32 In this series of patients, three became reinfected—two with new organisms and one with the same organism.
Using the articulating Prostalac antibiotic-loaded spacer has an infection cure rate of 93% (45/48 patients).32 In this series of patients, three became reinfected—two with new organisms and one with the same organism.
 The use of the articulating spacer allows patients to be more functional and thereby reduces the urgency to proceed with reimplantation. This delay between resection and reimplantation allows the surgeon to monitor the patient and assess for possible recurrence after the antibiotics have been stopped.
The use of the articulating spacer allows patients to be more functional and thereby reduces the urgency to proceed with reimplantation. This delay between resection and reimplantation allows the surgeon to monitor the patient and assess for possible recurrence after the antibiotics have been stopped.
 The optimal time from resection to reimplantation remains controversial; however, the longer the patient remains clinically free of infection between insertion of the spacer and reimplantation, the more likely that the infection has been cured.
The optimal time from resection to reimplantation remains controversial; however, the longer the patient remains clinically free of infection between insertion of the spacer and reimplantation, the more likely that the infection has been cured.
COMPLICATIONS
 General medical complications are similar to those of other revision procedures (eg, thromboembolic disease, postoperative ileus, or cardiac ischemia) and will not be further detailed.
General medical complications are similar to those of other revision procedures (eg, thromboembolic disease, postoperative ileus, or cardiac ischemia) and will not be further detailed.
 Local complications can occur with removal of the implants or with the spacer.
Local complications can occur with removal of the implants or with the spacer.
Removal of the implants, particularly well-fixed implants (as in other revision procedures), can result in bone loss, fracture, or canal perforation. These complications are not reported to be any greater in septic versus aseptic revisions.
 Complications related to the spacer depend on the type of spacer used.
Complications related to the spacer depend on the type of spacer used.
Static spacers, in addition to functional problems experienced by the patient, can result in difficulties at the time of reimplantation because of contractures or excessive shortening. Excessive shortening may make reestablishment of leg lengths more difficult.
Articulating spacers may cause polishing or sclerosis of the endosteum, resulting in bone that is less suitable for cementing should a cemented reconstruction be chosen at the time of reimplantation. However, cementless reconstruction is widely used and is not associated with an increased risk of infection. We rarely use cemented femoral reconstruction at the time of reimplantation and reserve its use for very low-demand patients with limited life expectancy.
Articulating spacers, as with conventional hip replacements, can lead to hip instability. This may be more common if there is bony or soft tissue deficiency. A snap-fit polyethylene liner used in the Prostalac system can markedly reduce this problem.
 Complications of the infection are failure to cure the infection and side effects or toxicity related to antibiotic use. Although there is some variation in the literature, it can be concluded that the rates for curing the infection are about 89% to 93%.4,7,8,17,31,32
Complications of the infection are failure to cure the infection and side effects or toxicity related to antibiotic use. Although there is some variation in the literature, it can be concluded that the rates for curing the infection are about 89% to 93%.4,7,8,17,31,32
The ability to cure the infection relates to a number of factors: the status of the local soft tissues, systemic comorbidities, the virulence of the organism, and surgical technique.
 The surgeon can improve outcomes by identifying the organism, performing a thorough débridement, and using appropriate high-dose antibiotics in the spacer. As noted earlier, the dose of antibiotics in cement may require adjustment in patients with renal insufficiency. Proper medical and nutritional support may also improve the outcome. Depending on which systemic antibiotics are used, monitoring serum levels is required to avoid toxicity.
The surgeon can improve outcomes by identifying the organism, performing a thorough débridement, and using appropriate high-dose antibiotics in the spacer. As noted earlier, the dose of antibiotics in cement may require adjustment in patients with renal insufficiency. Proper medical and nutritional support may also improve the outcome. Depending on which systemic antibiotics are used, monitoring serum levels is required to avoid toxicity.
REFERENCES
1. American Academy of Orthopaedic Surgeons. Clinical practice guidelines on the diagnosis of periprosthetic joint infections of the hip and knee. http://www.aaos.org/research/guidelines/PJIguideline.asp. Published June 18, 2010. Accessed February 11, 2014.
2. Barrack RL, Jennings RW, Wolfe MW, et al. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res 1997;345:8–16.
3. Ben-Lulu O, Farno A, Gross AE, et al. A modified cement spacer technique for infected total hip arthroplasties with significant bone loss. J Arthroplasty 2012;27(4):613–619.
4. Biring GS, Kostamo T, Garbuz DS, et al. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10 to 15 year follow-up study. J Bone Joint Surg Br 2009;91:1431–1437.
5. Duncan CP, Beauchamp C. A temporary antibiotic-loaded joint replacement system for management of complex infections involving the hip. Orthop Clin North Am 1993;24:751–759.
6. Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. J Bone Joint Surg Am 1994;76A:1742–1751.
7. Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty 1999;14:175–181.
8. Haddad FS, Muirhead-Allwood SK, Manktelow AR, et al. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br 2000;82B:689–694.
9. Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Relat Res 2002;403:16–22.
10. Hanssen AD, Spangehl MJ. Treatment of the infected hip replacement. Clin Orthop Relat Res 2004;420:63–71.
11. Hsieh PH, Shih CH, Chang YH, et al. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am 2004;86A:1989–1997.
12. Isiklar ZU, Demirors H, Akpinar S, et al. Two-stage treatment of chronic staphylococcal orthopaedic implant-related infections using vancomycin-impregnated PMMA spacer and rifampin-containing antibiotic protocol. Bull Hosp Jt Dis 1999;58:79–85.
13. Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg 2003;11:38–47.
14. Kuzyk PR, Dhotar HS, Sternheim A, et al. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: technique, controversies and outcomes. J Am Acad Orthop Surg 2014;22:153–164.
15. Mason JB, Fehring TK, Odum SM, et al. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty 2003;18:1038–1043.
16. Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty 1998;13:331–338.
17. Masri BA, Panagiotopoulos KP, Greidanus NV, et al. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty 2007;22:72–78.
18. Mirra JM, Amstutz HC, Matos M, et al. The pathology of joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res 1976;117:221–240.
19. Mitchell PA, Masri BA, Garbuz DS, et al. Cementless revision for infection following total hip arthroplasty. Instr Course Lect 2003;52:323–330.
20. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic infection. From the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992–2994.
21. Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic loaded CMW and Palacos-R bone cements. J Arthroplasty 1999;14:1141–1145.
22. Sanzen L, Sundberg M. Periprosthetic low-grade infection hip infections: erythrocyte sedimentation rate and C-reactive protein in 23 cases. Acta Orthop Scand 1997;68:461–465.
23. Schinsky MF, Della Valle CJ, Sporer SM, et al. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am 2008;90(9):1869–1875.
24. Shukla SK, Ward JP, Jacofsky MC, et al. Perioperative testing for persistent sepsis following resection arthroplasty of the hip for periprosthetic infection. J Arthroplasty 2010;25(6)(suppl 1):87–91.
25. Spangehl MJ, Masri BA, O’Connell JX, et al. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am 1999;81A:672–683.
26. Spangehl MJ, Masterson E, Masri BA, et al. The role of intraoperative Gram stain in the diagnosis of infection during revision total hip arthroplasty. J Arthroplasty 1999;14:952–956.
27. Spangehl MJ, Younger AS, Masri BA, et al. Diagnosis of infection following total hip arthroplasty. Instr Course Lect 1998;47:285–295.
28. Toms AD, Davidson D, Masri BA, et al. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br 2006;88B:149–155.
29. Trampuz A, Hanssen AD, Osman DR, et al. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 2004;117:556–562.
30. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996;78A:512–523.
31. Wentworth SJ, Masri BA, Duncan CP, et al. Hip prosthesis of antibiotic-loaded acrylic cement for the treatment of infections following total hip arthroplasty. J Bone Joint Surg Am 2002;84A(suppl 2):123–128.
32. Younger AS, Duncan CP, Masri B, et al. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty 1997;12:615–623.
33. Zmistowski B, Della Valle C, Bauer TW, et al. Workgroup 7: diagnosis of periprosthetic joint infection. In: Parvizi J, Gehrke T, eds. Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection. Brooklandville, MD: Data Trace Publishing, 2013:158.
< div class='tao-gold-member'>