Antiangiogenic Agents: Introduction
|
In 1971, Judah Folkman published a landmark paper hypothesizing that all tumor growth is dependent on angiogenesis and that inhibitors of angiogenesis could be used to treat cancers.1 The ensuing years have proven him correct and have seen the development of new agents that, either alone or in adjunct, have shown promise not only in oncology but in a variety of dermatologic conditions as well.
Antiangiogenic drugs can be classified as either “direct” or “indirect,” the former acting directly on untransformed endothelial cells to prevent proliferation, migration, or survival, a process that normally occurs upon stimulation by proangiogenic molecules; and the latter by inhibition of tumor-produced oncogenic protein products that promote proangiogenic states. Angiogenesis inhibitors as a drug class provide a unique approach to cancer treatment because they are also effective against slow-growing tumors, while traditional therapies, such as chemotherapy and radiation, work best on rapidly dividing cells. In the future, the switch to an angiogenic phenotype may be able to be blocked in clinically undetectable cancers, thus preventing disease progression using therapies directed, in part, by angiogenesis biomarkers.2,3 Current applications of these agents center on oncologic and ophthalmologic diseases but dermatologic indications are also promising.
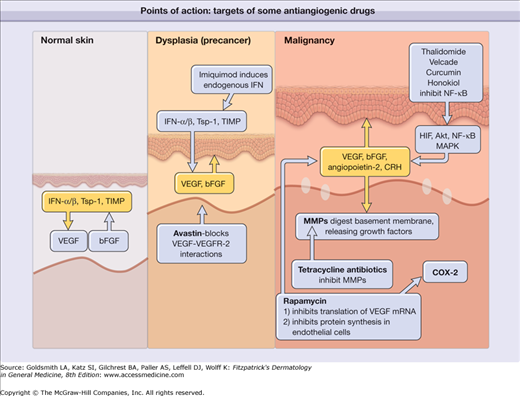
Figure 235-1 shows the key points of action of some of the antiangiogenic drugs discussed in this chapter in normal skin, inflammatory conditions, precancerous lesions, and malignancy.
Figure 235-1
Schematic depiction of the key points of action of some of the antiangiogenic drugs discussed in this chapter in normal skin, precancerous lesions, and malignancy. bFGF = basic fibroblast growth factor; COX-2 = cyclooxygenase 2; CRH = corticotropin-releasing hormone; HIF = histoplasma inhibitory factor; IFN = interferon; MAPK = mitogen-activated protein kinase; MMP = matrix metalloproteinase; mRNA = messenger RNA; NF-κB = nuclear factor κB; TIMP = tissue inhibitor of metalloproteinases; Tsp-1 = thrombospondin 1; VEGF = vascular endothelial growth factor; VEGFR-2 = vascular endothelial growth factor receptor 2.
Interferon-α 2B (Intron A)
Interferon-α (IFN-α) made history in 1988 as the first antiangiogenic therapy used in humans, for the successful treatment of pulmonary hemangiomatosis in a pediatric patient. IFN-α 2b is a synthetic cytokine made from the bacterium Escherichia coli transformed with recombinant DNA, and has similar actions to its natural endogenous counterpart, IFN-α, a type I IFN produced naturally by the immune system.
The IFNs act through the Janus kinase-signal transducers and activators of transcription (Jak-STAT) pathway. IFN-α, which is both a direct and indirect antiangiogenic agent, was first observed to impair capillary endothelial cell migration. Its properties as an indirect antiangiogenic agent include the ability to decrease tumor cell production of basic fibroblast growth factor (bFGF), which may explain its success in treating hemangiomata.3 IFN-α has well-known antiviral activity and antitumor activity, which may be mediated in part by upregulating major histocompatibility complex class I antigen expression, activating natural killer cells, controlling progression through cell cycle checkpoints, and activating apoptosis.
Interferon-α 2B is currently indicated for the treatment of chronic hepatitis B (which may be chemopreventive against hepatocellular carcinoma), chronic hepatitis C, acquired immunodeficiency syndrome (AIDS)-associated Kaposi sarcoma, and condylomata acuminata. It is also indicated in the following malignancies: malignant melanoma (adjuvant to surgical therapy in patients at high risk of systemic recurrence; must be administered within 56 days postoperatively), hairy cell leukemia, and follicular lymphoma.4 Off-label uses in dermatology include cutaneous T-cell lymphoma (CTCL, mycosis fungoides) and basal and squamous cell skin cancer. It has also been used in the treatment of infantile hemangiomas along with corticosteroids or in the event of corticosteroid resistance; however, given rising concerns over the risk of spastic diplegia (especially in children under 1 year of age), its utility in this setting has been somewhat tempered.5
(See Box 235-1)
|
IFN-α 2b comes as a powder that, upon reconstitution, should be used immediately but may be stored up to 24 hours at 2°C–8°C (36°F–46°F). It can be given by subcutaneous (SC), intramuscular (IM), intravenous (IV), or intralesional routes. Its half-life is 3–12 hours when given SC or IM and 30 minutes when given IV. The drug is likely metabolized by the kidneys. Polyethylene glycol IFN-α 2b has a 10-fold increase in the half life, decreased toxicity, and increased compliance.4,6 For dosing, see Table 235-1.
Disease | Dose |
|---|---|
Malignant melanoma high-dose regimen | Induction: 20 million IU/m2 IV infusion over 20 minutes, 5 consecutive days/week for 4 weeks Maintenance: 10 million IU/m2 SC three times/week for 48 weeks |
Malignant melanoma adjuvant low-dose regimen (in clinical trials) | Maintenance: 3 million U SC three times/week for 1 year |
Acquired immunodeficiency syndrome-related Kaposi sarcoma | 30 million IU/m2 SC or IM three times/week until disease progression or maximal response has been achieved after 16 weeks |
Condylomata acuminata | 1 million IU per lesion (maximum of five lesions per course) three times/week on alternate days for 3 weeks. May administer additional courses at 12–16-week intervals |
Hemangiomas | 3 million U/m2/day for at least 6 months.7 |
(See Box 235-2)
|
Hemoglobin, complete blood cell count with differential, electrolytes, liver function tests, thyroid-stimulating hormone, chest X-ray, and eye examination should be obtained before initiation of and during therapy on a routine basis. Patients with prior cardiac disease or advanced cancer require a baseline electrocardiogram and routine reevaluation thereafter. Therapy should not be initiated in patients with a history of depression or severe psychiatric disorder due to the risk of worsening psychiatric symptoms and suicide. Patients with thyroid disorders that cannot be corrected with medication should not be treated. The development of spastic diplegia in patients with hemangiomas taking IFN seems to correlate with age and severe hypothyroidism (due to the presence of ectopic thyroxin degrading enzymes produced by the hemangiomas). Thus, thyroid monitoring is mandatory in all patients with large hemangiomas.4,5
Treatment should be discontinued if the absolute neutrophil count (ANC) reaches less than 0.5 × 109/L or the platelet count reaches less than 25 × 109/L. Other symptoms warranting discontinuation include new-onset or worsening eye symptoms, the development of thyroid dysfunction uncorrectable by medication, and the development of severe depression or other psychiatric disorder during therapy.4
Bevacizumab (Avastin)
Bevacizumab is a recombinant, humanized, immunoglobulin G1 monoclonal antibody against vascular endothelial growth factor (VEGF). VEGF is a growth factor that promotes angiogenesis by supporting endothelial cell replication and survival as well as vascular permeability. This potent proangiogenic molecule is upregulated in a majority of human tumors and serves to alter tumor vasculature.
Binding of bevacizumab to VEGF is direct and specific. The antibody also competitively binds VEGF receptor 1 (VEGFR-1) and VEGFR-2, preventing VEGF from binding to its receptors and initiating the signaling cascade. It has also been shown to reduce and normalize tumor vascularity.8
Bevacizumab was the first antiangiogenesis drug approved by the US Food and Drug Administration (FDA) in February 2004 for the first-line treatment of metastatic colorectal carcinoma when given in combination with intravenous 5-fluorouracil-based chemotherapy but also has indications for nonsmall cell lung cancer, metastatic human epithelial growth factor-2-negative breast cancer, glioblastoma, and metastatic renal cell carcinoma.9 Bevacizumab, in combination with either other antiangiogenic agents or chemotherapy, is currently being investigated in metastatic melanoma, hemangioendothelioma, and angiosarcoma. Other than a history of hypersensitivity, no known contraindications to bevacizumab therapy exist.9
(See Box 235-3)
|
 Ranibizumab (Lucentis)
Ranibizumab (Lucentis)
Ranibizumab is an antibody fragment (FAb) related to bevacizumab that also competitively binds VEGF and inhibits its interactions with VEGFR-1 and -2; it acts to block endothelial proliferation and survival. Smaller in size than bevacizumab (a full-length antibody), ranibizumab is thought to have better tissue absorption and fewer inflammatory reactions.10
Ranibizumab is currently indicated for the treatment of patients with neovascular, or wet, age-related macular degeneration; however, clinical trials looking into its use in cutaneous neurofibroma and port wine stain in combination with pulsed dye laser are ongoing.11
 Pegaptanib (Macugen)
Pegaptanib (Macugen)
Pegaptanib is a short peptide strand, or aptamer, directed against a specific isomer of secreted VEGF, VEGF165; VEGF165 is thought to play a particular role in endothelial cell proliferation.12
Like ranibizumab, pegaptanib is currently indicated for the treatment of neovascular, or wet, age-related macular degeneration; however, no known dermatologic uses or investigations into potential uses exist at this time.13
- Endophthalmitis, retinal detachment, and traumatic cataract formation associated with intravitreal administration13
Cetuximab (Erbitux)
Cetuximab is a chimeric, human-murine monoclonal antibody against the epithelial growth factor receptor (EGFR), and, as such, competitively inhibits the binding of epithelial growth factor (EGF), transforming growth factor (TGF), and other associated ligands. Downstream signaling blockade results in myriad of antitumor properties including decreased proliferation, cellular motility, and invasive potential via downregulation of signaling molecules such as basic fibroblast growth factor (bFGF, a product of keratinocytes) and interleukin 8 (IL-8).14,15 Angiogenesis is also inhibited through decreased EGFR-mediated VEGF expression.
Cetuximab is currently indicated for the treatment of metastatic colorectal carcinoma and squamous cell carcinoma of the head and neck, either alone or in combination with radiation or chemotherapy.16 Additionally, its use in squamous cell carcinoma of the skin is currently under investigation. Given the demonstrated role of bFGF in melanoma, cetuximab may also play an eventual role in its treatment. Other than a history of hypersensitivity, there are no known contraindications.
Panitumumab (Vectibix)
Panitumumab is an entirely human monoclonal antibody directed against EGFR. Like cetuximab, it blocks ligand interactions associated with the EGFR (EGF, TGF) and downstream signaling molecule like VEGF, bFGF, and IL-8; however, because panitumumab is not chimeric, the hypothesized benefit is better overall efficacy and immunologic tolerance.17
Panitumumab is currently indicated for progressing, metastatic colorectal carcinoma in combination with or as a single agent following chemotherapy.18 No known dermatologic uses or investigations into potential uses exist at this time, but, given its similarity to cetuximab, possible investigations into panitumumab’s utility in cutaneous squamous cell carcinoma and melanoma is warranted. Other than a history of hypersensitivity, there are no known contraindications.
 Trastuzumab (Herceptin)
Trastuzumab (Herceptin)
Trastuzumab is a human, monoclonal antibody directed against the human estrogen receptor 2 (HER-2). Blockade of HER-2 is results in downregulation of angiopoeitin-1 (Ang-1), plasminogen-activator inhibitor-1, VEGF, and TFG-α but upregulation of thrombospondin-1 (TSP-1), an inhibitor of angiogenesis.19
Trastuzumab is currently indicated for the treatment of HER-2-overexpressing, lymph node-positive breast cancer or breast cancer that is node-negative and is estrogen receptor/progesterone receptor-negative or has one high risk feature.20 No known dermatologic uses or investigations into potential uses exist at this time. Other than a history of hypersensitivity, there are no known contraindications.
 Sunitinib (Sutent)
Sunitinib (Sutent)
Sunitinib is a small-molecule, phosphorylation inhibitor of several tyrosine kinase receptors (RTK), including platelet-derived growth factor receptor-α (PDGF-α) and PDGF-β and VEGFR-1, 2, and 3; tumor growth inhibition and regression ensues by a variety of downstream mechanisms.21
Sunitinib is currently indicated for the treatment of gastrointestinal stromal tumors and advanced renal cell carcinoma.22 Clinical trials are ongoing as to the efficacy of sunitinib in metastatic melanoma, but case reports exist if its efficacy in this condition as well as skin ulcers associated with angioosteohypertrophy syndrome (or Klippel-Trénaunay syndrome, hemangiectatic hypertrophy).23 Other than a history of hypersensitivity, there are no known contraindications.
 Sorafenib (Nexavar)
Sorafenib (Nexavar)
Sorafenib is a small-molecule, phosphorylation inhibitor of several RTKs, including PDGF-β, VEGFR-1, 2, and 3, and RAF kinase.24,25 Like sunitinib, inhibition of multiple downstream RTK signaling results in tumor growth inhibition and regression.
Sorafenib is currently indicated for the treatment of advanced renal cell carcinoma and unresectable hepatocellular carcinoma.26 Ongoing clinical trials are looking into the use of sorafenib in the treatment of metastatic melanoma, both alone and in combination with chemotherapy (carboplatin and paclitaxel). Other than a history of hypersensitivity, there are no known contraindications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree