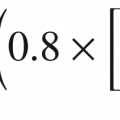Multimodal analgesia
25.2 Burn Pain Pathophysiology
The majority of burn patients experience different distinct and at times overlapping entities of pain pathophysiology concurrently and/or subsequently, explaining the need of advanced protocols providing multi-modal analgesia. The most common pain entities burn patients are experiencing include acute somatic pain, procedural pain, neuropathic pain, psycho-spiritual-emotional pain, and/or chronic persistent pain and will be discussed in more detail below.
25.3 Acute Somatic Pain
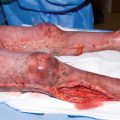
Acute somatic nociceptive pain is caused by the actual skin and tissue injury of the burn trauma as well as by the repetitive trauma (such as debridement, graft, or inflammation) during the initial hospitalization. The key to preventing long-lasting pain appears to initiate “multi-modal analgesia” [18] pain protocols on day one of the burn injury. Studies have shown that if pain is not adequately controlled right after the burn trauma, there is an increased risk of post-traumatic stress disorder (PTSD) in infants, children, and adults [3–6].
- 1.
Basic analgesia: acetaminophen (paracetamol) plus a non-steroidal-anti-inflammatory drug (NSAIDs), such as ibuprofen or ketorolac. If bleeding side-effects or stomach discomfort occurs, another option might be a COX-2 inhibitor, such as celecoxib.
- 2.
Opioids: include medications such as tramadol, morphine, fentanyl, hydromorphone, oxycodone, or methadone carefully titrated to effect. However, “If Coke doesn’t work, switch to Pepsi”—in other words, a significant number of children or adults may experience opioid-induced side-effect (which might be mitigated by a low-dose naloxone infusion) or poor analgesia on one opioid and then need to be “rotated” or switched to another strong opioid for better control. After discharge to home we wean slowly the opioids completely off and in the absence of new tissue trauma hardly ever expect patient to be on opioids for a long time (e.g., not longer than 1–2 months at home).
- 3.
Adjuvant Analgesia: such as gabapentinoids (e.g., gabapentin, pregabalin), alpha-agonist (e.g., dexmedetomidine, clonidine), NMDA-channel blocker (low-dose ketamine, methadone), sodium-channel blocker (e.g., lidocaine), tricyclic antidepressants (e.g., low-dose amitriptyline).
- 4.
Interventional modalities or neuroaxial analgesia (e.g., nerve blocks, paravertebral blocks, or epidural pain pumps).
- 5.
Rehabilitation: Physical therapy, occupational therapy, speech therapy.
- 6.
Psychology, stress-reduction.
- 7.
Active integrative (“non-pharmacological”) therapies—treatments/remedies that do not involve the use of medications, such as active mind–body techniques (deep breathing, biofeedback , self-hypnosis, etc.)
Procedural pain might be caused by dressing changes, intravenous (IV) access, blood draws, injections, etc. Patients report that in addition to dressing changes especially repetitive needle pokes are among the worst kind of pain they experience during their hospitalization [12]. Although this kind of pain can be completely prevented or significantly reduced by simple strategies, many hospitals may not be offering these strategies to all their patients yet.
“Numb the skin” (for children 36 weeks corrected gestational age and older) 4% lidocaine cream [22] or needle-less lidocaine application via a J-tip® (sterile, single-use, disposable injector that uses pressurized gas to propel medication through the skin) [23, 24] as topical anesthetics.
Sucrose [25, 26] or breastfeeding [27] for infants 0–12 months [28].
Comfort positioning. Restraining children for procedures is never supportive and creates a negative experience. Children restrained for painful procedures reported they felt ashamed, humiliated, powerless, and described the loss of the right to control their own body [29]. For infants swaddling, warmth, skin-to-skin contact, or facilitated tucking. For children who are 6 months and older offer them to sit upright, including on their parent’s lap.
Age-appropriate distraction [30], such as toys, books, blowing bubbles or pinwheels, stress balls, and using apps, videos, or games on electronic devices.
Neuropathic pain is defined by the International Association for the Study of Pain (IASP) as pain arising as a direct consequence of a lesion or disease affecting the “somatosensory” (i.e., nervous) system [31]. A significant number of burn patients develop neuropathic pain as a result of nerve damage caused by the burn trauma and the treatment [32]. In addition to NSAIDs and opioids (for the initial post-traumatic hospitalization only), several “adjuvant” pain medications, including gabapentinoids, low-dose tricyclic antidepressants; alpha-agonists and/or NMDA-channel blocker are commonly administered to mitigate pain. Although several medications may assist with controlling neuropathic pain, physical therapy, and psychology (and for some patients: nerve blocks) are usually required components of excellent pain control and should not be omitted.
Psycho-spiritual-emotional pain . The psychological and emotional impact of a burn injury [33] results in real existing measurable pain—however, this pain cannot be treated by opioids (or other pain medications), but rather by addressing those needs through family and social support as well as an interdisciplinary care team which includes team members such as a social worker, chaplain, and/or psychologist.
Chronic or persistent pain: Pain can persist after healing, with many patients after severe burns reporting ongoing burn-related pain many years later. In one large study [34], 358 burn survivors with injuries covering an average of 59% of their bodies were asked about their pain experience on average 12 years after the trauma: The majority (52%) described ongoing burn-related pain, two-thirds (66%) reported that pain interfered with their rehabilitation, and 55% reported that pain interfered with their daily lives.
Physical therapy/exercise: Many patients with chronic pain are deconditioned and exercise may even cause worsening of pain. A thoughtful daily (at home) training program then is required to improve movement and normalize function as much as possible.
Active integrative therapies, such as daily practicing of deep breathing, biofeedback (a technique using a video-game that trains people to improve their pain by controlling relaxing bodily processes that normally happen involuntarily, such as heart rate, blood pressure, muscle tension, and skin temperature.), self-hypnosis, mindfulness, progressive muscle relaxation, and/or yoga can reduce pain by stimulating “endorphins” (the body’s own pain medication that makes us to feel good) in the pain center of the brain.
Psychology: Pain can cause stress, and stress usually worsens pain. Worsened pain then worsens mood, which may affect anxiety and depression. Effective strategies include cognitive behavioral therapy (CBT), or play therapy for children, and stress-reduction offered by a licensed therapist.
Normalizing Life: Key to effective pain control appears to normalize function first, and then the pain gets better (unfortunately not the other way around), including returning to school or work, normalizing sleep, normalizing exercise and social life.
Medications are usually ineffective for a large number of patients with chronic and persistent pain, if not accompanied by the above four strategies. Opioids are usually not indicated for chronic persistent pain (unless there is repetitive new tissue injury) [36]. Some adjuvant analgesia, especially for nerve pain, however appear to be well tolerated and might be effective, as described below.
25.4 Regional Anesthesia
The majority of burn pain patients to date unfortunately do not receive one of the most effective analgesic modalities, which would prevent and treat unrelieved pain with the least amount side-effects: Regional or neuroaxial anesthesia [37–42]. Patients often have more intense postoperative pain from the split-thickness skin donor site than from the grafted burn wound [42, 43]. Especially if a burn injury of an extremity or the trunk requires hospitalization on a burn unit, it must now be expected standard of care to ensure assessment the infant, child, adolescent, or adult by an anesthesiologist for potential regional anesthesia. Blocking pain nociception using a local anesthetic such as bupivacaine, in some cases in conjunction with an opioid and/or alpha-agonist can provide complete analgesia, without any of the opioid-induced side-effects. Pain pathways can be blocked, when anesthesiologist trained in regional anesthesia utilize central neuraxial infusions, peripheral nerve and plexus blocks or infusions, or neurolytic blocks [44]. Occasionally, implanted intrathecal ports and pumps for baclofen, opioids, local anesthetics, and other adjuvants might be considered.
significantly reduce or eliminated need for opioids
no systemic side-effects
no sedation
no nausea
minimal side-effects with epidural (itching, urinary retention)
improved gastrointestinal motility
less postoperative cardiac arrhythmias
significantly reduced pulmonary complications
significantly reduced delirium
improved mobility that reduces rates of deep vein thrombosis (DVTs)
extremely high patient satisfaction
patient is awake and can remember conversations with clinicians and family
evidence for reduction of development of chronic pain and phantom pain
Central neuraxial techniques (spinal and epidural catheters) have been utilized with good effect as both primary anesthetics and postoperative adjuncts in burn-injured patients [43]. Epidural abscesses are not more common in burn patients, but there might be an increased risk that intravascular catheters are more likely to become infected if placed in or near burned tissue [42], so similarly caution is likely reasonable in selecting appropriate burn patients for central neuraxial techniques [43].
Because the nociceptive nerves cannot be numbed independent of all the other nerves that receive local anesthesia (“what wires together, fires together”), there are side-effects such as motor weakness, hypotension, pruritus, or urinary retention [45]. If the patient has breakthrough pain that breaks through a low continuous infusion of the local anesthetic, a patient-controlled analgesia (PCA) bolus allows the patient to give him- or herself additional medication as needed, called patient-controlled regional analgesia (PCRA). Similar to an opioid PCA, the patient can use their PCRA button for breakthrough pain, but without the side-effects caused by opioids. Patients can be sent home with a nerve block catheter, connected to a disposable pump or one that is returned to the hospital. There are no opioids in the infusion, eliminating misuse potential. That may lead to less adverse events, including sedation, delirium, sleep disturbances, and opioid-induced hyperalgesia.
25.5 Pharmacological Considerations
Large burns in children and adults result in altered pharmacokinetic and pharmacodynamic responses to many medications. Plasma protein loss through injured skin and further dilution of plasma proteins by resuscitation fluids decrease the concentration of albumin, an important drug-binding protein [43]. There is an increase in volume of distribution in most studied medications, including opioids such as fentanyl, the general anesthetic propofol, and muscle relaxants [46]. During the acute injury (resuscitation), phase of large burns in the first 48 h cardiac output, and subsequently renal and hepatic blood flow is decreased, possibly increasing half-life of opioids and other analgesics and requiring somewhat lower starting doses or frequency of administration. However, around day three during the hyperdynamic phase elevated renal and hepatic blood flow results in increased clearance, and doses of analgesics (and sedatives) commonly need to be increased significantly [43].
Morphine, for instance, is metabolized by the liver glucuronyl-transferase into morphine-6 glucuronide (M6G) and morphine-3 glucuronide (M3G). M6G is a much stronger analgesic (40–100 times stronger) and displays adverse effects including nausea, vomiting, sedation, and respiratory depression. M3G is not an analgesic but is a μ-opioid antidote with unique adverse effects, especially hyperexcitability and neurotoxicity. The ratio of M6G/M3G thereby defines the analgesia to adverse effect profile in individual patients. Both metabolites need to be excreted by the kidney, and patients in renal failure, and/or during low cardiac output of the resuscitation phase 0–48 h with subsequently decreased renal and hepatic blood, have a higher risk of unwanted side-effects. Fentanyl or methadone, neither of which is excreted renally, might be a better choice in this scenario.
25.6 Pharmacology Step 1: Basic Analgesia
Acetaminophen (Paracetamol) (10–15 mg/kg p.o./p.r./i.v. every 4–6 h; dose limit: <2 years: 40 mg/kg/day, >2 years : 75 mg/kg/day, max. 650 mg every 6 h) is generally well tolerated by children and adults and lacks gastrointestinal and hematological side-effects. Significant hepatoxicity [47] is rare, but careful attention to dosing is paramount.
Ibuprofen (5–10 mg/kg p.o. every 6 h; dose limit 2400 mg/day) has the least gastrointestinal side-effects among non-steroidal anti-inflammatory drugs (NSAIDs) that are nonselective for cyclooxygenase-2 (COX-2). It should be used with caution in individuals with hepatic or renal impairment, or a history of gastrointestinal bleeding or ulcers, and it inhibits platelet aggregation.
Ibuprofen-sodium Meta-analysis showed that NSAID-salts display far more rapid absorption, faster initial pain reduction, good overall analgesia in more patients at the same dose, and probably evoke longer-lasting analgesia, without reports of adverse events [48]. When compared to ibuprofen, ibuprofen-sodium (available over the counter in the United States and many countries) produces significantly greater analgesia over 6 h, and required fewer re-medications than standard formulations [49]. In addition, 200 mg fast-acting ibuprofen (Numbers-needed-to-treat [NNT] 2.1; 95% confidence interval (CI) 1.9–2.4) was as effective as 400 mg standard ibuprofen (NNT 2.4; 95% CI 2.2–2.5), and produced a faster onset of analgesia.
Ketorolac has the advantage of i.v. administration, but it should be rotated to oral ibuprofen, as soon as tolerated (<2 years: 0.25 mg/kg every 6 h; >2 years: 0.5 mg/kg every 6 h; max. 30 mg/dose; recommended dosing no longer than 3–5 days).
Celecoxib (a COX-2 > COX-1 inhibitor) might be considered if classical NSAIDs are contraindicated (e.g., owing to bleeding risks, or gastrointestinal side-effects). It does not display less renal toxicity compared to classic NSAIDs. Safety and efficacy have been established only in children 2 years of age or older and for a maximum of 6 months of treatment in juvenile rheumatoid arthritis (1–2 mg/dose [max. 100 mg] every 12 h).
Basic analgesia for children (>6 months) and adults
Drug | Route | Pediatric dose | Maximal dose | Dosing interval |
|---|---|---|---|---|
Ibuprofen | PO | 5–10 mg/kg | 400–600 mg | 6–8 h |
Ibuprofen-sodiuma (Advil®) 256 mg tablet = 200 mg ibuprofen | PO | 5–10 mg/kg | 200–400mg | 6–8 h |
Acetaminophen | PO, PR | 10–15 mg/kg | 60 mg/kg/day <2 years 90 mg/kg/day >2 years | 4–6 h |
Acetaminophenb | IV | <10 kg = 7.5 mg/kg; | 30 mg/kg/day | 6 h |
1–2 years = 15 mg/kg; | 60 mg/kg/day | 6 h | ||
>2 years (<50 kg) = 15 mg/kg; | 75 mg/kg/day | 6 h | ||
>13 years (>50 kg) = 1000 mg | 4000 mg/day | 6 h | ||
Ketorolacc (Toradol) | IV | <2 years = 0.25 mg/kg >2 years = 0.5 mg/kg | 30 mg | 6–8 h |
Celecoxibd | PO | 1–2 mg/kg | 100 mg | 12–24 h |
25.7 Pharmacology Step 2: Opioids
Opioids remain a mainstay in the analgesic treatment of acute somatic pain cause by the tissue injury as well as subsequent interventions, including pain at skin donor site and the grafted burn wound. Opioid rotation may be necessary, if tolerance develops or dose-limiting opioid toxicity occurs. A switch from one opioid to another is often accompanied by a change in the balance between analgesia and side-effects [50]. A favorable change in opioid side-effect profile may be experienced if there is less cross-tolerance at the opioid receptors mediating analgesia than at those mediating adverse effects. If rotating opioids because of decreasing effectiveness or limiting side-effects (i.e., because of incomplete cross-tolerance), it can be considered to begin at around 50% of the equianalgesic dose and titrate to effect. However, the required decrease for incomplete cross-tolerance may be higher or lower, depending on the clinical context of the individual patient [51]. Opioid-associated side-effects (e.g., constipation, pruritus, and nausea) should be anticipated and treated accordingly.
Opioid analgesics: usual starting doses for children (>6 months) and adults
Drug (route of administration) | Equianalgesic dose (parenteral) | Starting dose IV | IV:PO ratio | Starting dose PO (transdermal) |
|---|---|---|---|---|
Morphine (PO, SL, IV, SC, PR) | 10 mg | Bolus dose: 0.05–0.1 mg/kg (max. 5 mg) every 2–4 h Continuous infusion: 0.01–0.03 mg/kg/h (max. 0.5–1.5 mg/h) | 1:3 | 0.15–0.3 mg/kg (max. 7.5–15 mg) every 4 h |
Fentanyl (IV, SC, SL, transdermal, buccal) | 100–250 μg | Bolus dose: 1–3 μg/kg (max. 25–75 μg) (slowly over 3–5 min—fast bolus of higher doses may cause thorax rigidity) Continuous infusion: 1–2 μg/kg/h (max. 50–100 μg/h) | 1:1 (IV to trans dermal) | 12 μg/h patch (must be on the equivalent of at least 30 mg oral morphine/24 h, before switched to patch) |
Hydromorphone (PO, SL, IV, SC, PR) | 1.5–2 mg | Bolus dose: 15–20 μg/kg (max. 1 mg) every 4 h Continuous infusion: 5 μg/kg/h (max. 250 μg/h) | 1:5 | 60 μg/kg (max. 2000–3000 μg or 2–3 mg) every 3–4 h |
Oxycodone (PO, SL, PR) | 5–10 mg | n/a | n/a | 0.1–0.2 mg/kg (max. 5–10 mg) every 4 h or 0.15–0.3 mg/kg (max. 7.5–15 mg) every 6 h |
Tramadol (PO, PR) | 100 mg | IV not available in the United States [Bolus dose: 1 mg/kg every 3–4 h Continuous infusion: 0.25 mg/kg/h] | 1:1 | 1–2 mg/kg every 3–4 h, max. of 8 mg/kg/day (>50 kg: max. of 400 mg/day) |
Methadone (PO, PR, SL, IV) | Nonlinear conversion (see Table 25.5) | 0.04–0.08 mg/kg (max. 2–4 mg) IV Q8h | 1:1 to 1:2 (in adults usually IV usually 50% of PO dose; in pediatrics usually IV = 80% of PO dose) | 0.05–0.1 mg/kg (max. 2.2–5 mg) PO Q8h |
Drug | Route | Pediatric Dose (age) | Maximal Dose | Dosing Interval |
|---|---|---|---|---|
Basic Analgesia | ||||
Ibuprofena | PO | 5–10 mg/kg (infants 3–6 months) | 40 mg/kg/day | 6–8 h |
Acetaminophen | PO, PR | 5–10 mg/kg (neonates 0–30 days) 10 mg/kg (infants 1–3 months) 10–15 mg/kg (infants 3–6 months) | 20–40 mg/kg/day 40 mg/kg/day 40–60 mg/kg/day | 4–6 h (maximum 4 doses/day) |
Acetaminophenb | IV | <10 kg = 7.5 mg/kg | 30 mg/kg/day | 6 h |
Opioids | ||||
Morphine | PO/PR/SL | 0.075–0.15 mg (neonates 0–30 days) | 6 h | |
0.08–0.2 mg (infants 1–6 months) | 4–6 h | |||
Morphinec | IV/SCd | 0.025–0.05 mg/kg (neonates 0–30 days) | 6 h | |
0.1 mg/kg (infants 1–6 months) | 6 h | |||
Infusion (with PCA bolus of same dose): 0.005–0.01 mg/kg/h (neonates 0–30 days) 0.01–0.03 mg/kg/h(infants 1–6 months) | ||||
Fentanylc | IV/SCd | 1–2 μg/kg (neonates and infants 0–12 months) | 2–4 h | |
Infusion (with PCA bolus of same dose) : 0.5–1 μg/kg/h (neonates and infants 0–6 months) | ||||
Oxycodone | PO/PR/SL | 0.05–0.125 mg/kg (infants 1–6 months) | 4 h | |
Adjuvant Analgesics | ||||
Gabapentin | PO | 4.5 mg/kg (neonates and infants 0–6 months) | 15 mg/kg | 6 h |
Dexmedetomidine (Precedex®) | IV | Infusion: | Slowly titrate to max. of | Cont. infusion |
0.2 μg/kg/h (neonates and infants 0–6 months) | 2 μg/kg/h | |||
Clonidine | PO | 1–3 μg/kg | 4–6 h | |
Amitriptylinee | PO | 0.1 mg/kg (infants 3–12 months) | 0.4 mg/kg | QHS (once at night) |
Morphine appears safe and efficacious in full-term neonates; however, starting doses are usually lower compared to those used in older children (see Table 25.3). Long-term neurodevelopmental outcome years after former preterm or term babies are exposed to continuous morphine or fentanyl infusion displayed no adverse effects of the opioids on intelligence, motor function, or behavior [53–56].
Fentanyl is a popular opioid for analgesia prior to painful procedures owing to its rapid onset (about 1 min) and its brief duration of action (30–45 min). Intranasal fentanyl for dressing changes in burn patients [57]. Fentanyl provides a good alternative to morphine when tolerance or dose-limiting side-effects mandate opioid rotation [58, 59].
Hydromorphone, like morphine and fentanyl, is another selective μ-opioid receptor agonist. Unlike morphine metabolism, there is no hydromorphone-6-glucuronide (H6G), but metabolism of the parent compound does result in hydromorphone-3-glucoronide (H3G). Opioid hyperexcitability has been reported in patients with renal failure taking hydromorphone [60, 61]. The normal H3G to hydromorphone plasma ratio is 27:1, but in renal failure it is 100:1 [62].
Oxycodone is a selective μ-opioid receptor agonist although some animal studies suggest κ-receptor agonist activity as well [63] The oral potency ratio of oral oxycodone to morphine is between 1:1 and 2:1 [64]. One advantage of oxycodone over morphine is the slightly longer half-life, frequently 6-h dosing (as oppose to 4-h with morphine). Renal and hepatic impairment increase oxycodone serum levels [65].
Usual starting doses for patient (or nurse)-controlled analgesia (PCA) pumps—dose escalation usually in 50% increments both for continuous and PCA bolus dose (Department of Pain Medicine, Palliative Care & Integrative Medicine, Children’s Hospitals and Clinics of Minnesota, USA)
Continuous infusion [μg/kg/h] | PCA bolus [μg] | Lock-out time [min] | Maximum number of boluses/hour | |
|---|---|---|---|---|
Morphine | 20 (max. 1000) | 20 (max. 1000) | 5–10 | 4–6 |
Hydromorphone | 3–5 (max. 250) | 3–5 (max. 250) | 5–10 | 4–6 |
Fentanyl | 1 (max. 50) | 1 (max. 50) | 5 | 4–6 |
Methadone is an excellent opioid choice in advanced pediatric and adult analgesia, but it remains under-utilized [66–68]. In the United States, it is available as an intravenous formulation also (with commonly used conversion ratios of adults: 1 mg PO = 0.5 mg IV and 1 mg IV = 1 mg PO; however in Pediatrics: 1 mg PO = 0.8 mg IV and 1 mg IV = 1.2 mg PO). Early methadone initiation may reduce the development of opioid tolerance (i.e., reducing the need to increase opioids rapidly to achieve analgesia) and thereby have a significant effect on ventilator outcomes in critically injured patients with burn injury [69].
This multi-mechanistic analgesic is a μ (δ, κ)-opioid receptor agonist, an NMDA-channel blocker, and a presynaptic blocker of serotonin and norepinephrine re-uptake. Advantages include methadone’s long half-life (allowing every 8–12 h dosing), high effectiveness in complex pain conditions, including the management of neuropathic pain, decreased incidence of constipation, lack of active metabolites, and safe usage in renal failure and in stable liver disease.
There are several disadvantages, however, including wide dosing variation, a long half-life (which may lead to accumulation, making quick titration difficult), and a more complex equianalgesic conversion, which requires longer and closer patient observation than with other opioids. Safe use requires that the effects of methadone should be closely monitored for several days, particularly when it is first started and after any dosing change. Methadone has been effectively used in burn patients [6, 70].
Adjuvant analgesics used in pediatric and adult pain management (Pain Medicine and Palliative Care, Children’s Hospitals and Clinics of Minnesota) [2]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree