Dose–response curves for rocuronium in burned and non-burned adults. On the y-axis, the train-of-four (TOF) ratio is represented as the percentage of twitch suppression between the fourth and first twitch at 2 Hz. Burned adults with mean 40% TBSA burns, at least 1 week following the burn injury, were studied at 0.9, 1.2, and 1.5 mg/kg rocuronium. In controls (non-burned adults), rocuronium administration is shown at a dose of 0.9 mg/kg, resulting in a 95% twitch suppression in ≤60 s. In the burned adults, a similar dose required >120 s for 95% twitch suppression. A dose of 1.5 mg/kg still required >90 s for 95% twitch suppression
Pharmacokinetic alterations in burn injury
Medication class | Pharmacokinetic alteration in burn injury |
|---|---|
Depolarizing neuromuscular blockers | Increased sensitivity (contraindicated after 72 h) |
Non-depolarizing neuromuscular blockers | Resistance (seen usually after 72 h) |
Aminoglycosides | Increased clearance |
Benzodiazepines | Variable clearance depending on metabolic pathway |
H2-antagonists | Increased clearance |
Opioids | Increased clearance, increased volume of distribution (morphine and fentanyl) and unknown for methadone |
Dexmedetomidine, a selective α2-antagonist, may be used for sedation and analgesia in critically ill burn patients, as well as an adjunct agent during or premedication prior to a general anesthetic, particularly in opioid tolerant patients. A recent meta-analysis concludes that dexmedetomidine may provide deeper sedation and prevent hypertension in burn patients, but due to the known hypotensive potential, it is important to ensure euvolemia and limit total dosage administered to prevent hypotension [49]. Because the alterations in pharmacokinetics make the response to any medication somewhat unpredictable, clinical effects should be closely monitored, and guided by laboratory analysis of plasma concentration whenever possible.
24.3.2 Multimodal Sedation and Analgesia Guidelines
Examples of sedation and analgesia guidelines
Stage of injury | Baseline anxiolysis | Baseline analgesia | Procedural anxiolysis | Procedural analgesia |
|---|---|---|---|---|
Acute burn, mechanically ventilated | 1. Midazolam infusion 2. Dexmedetomidine infusion 3. Antipsychotics 4. Propofol infusion (use for <48 h) | Morphine or fentanyl infusion | 1. Midazolam boluses 2. Higher dexmedetomidine infusion 3. Slow haloperidol boluses 4. Propofol boluses | Morphine or fentanyl boluses |
Acute burn, not mechanically ventilated | Dexmedetomidine (IV) or scheduled lorazepam (IV or PO) | Morphine (IV or PO) or fentanyl (IV) | Lorazepam (IV or PO) | Morphine (IV or PO) or fentanyl (IV) or ketamine (IV) |
Chronic acute burn | Scheduled lorazepam (PO) or antipsychotics | Scheduled morphine or methadone | Lorazepam or antipsychotics | Morphine (PO) or oxycodone |
24.4 Anesthetic Care of the Burn Patient
24.4.1 Airway Management
Both acute injuries and chronic sequelae following burns may make airway management difficult. Factors that contribute to this difficulty include macroglossia or direct thermal injury to the glottis and airways during the acute phase, as well as limited mouth opening and neck motion due to contractures in the subacute to chronic phase [51]. Burn victims who sustained injury in a closed space have increased likelihood of airway injury. Signs of inhalational injury include vocal changes, stridor, or hoarseness, and these may be an important predictor of difficult intubation. Fiberoptic intubation may be used on the “awake” but sedated patient. Dexmedetomidine may be used to provide sedation without respiratory depression, while preventing large sympathetic responses to the procedure. For patients with macroglossia secondary to edema, manual distraction of the tongue and jaw lift can be helpful. Suction or gauze may be utilized to aid with grasping the tongue for such a maneuver [52]. Laryngeal mask airway may also be placed once general anesthesia is induced and used to ventilate the patient while the bronchoscope is guided through the LMA lumen. LMA-aided intubation may be especially useful in the case of perioral edema. Additional techniques include the use of retrograde wires following tracheotomy or tracheostomy, fiber-optic “stylets” that can fit through narrow mouth openings, as well as light-wand-guided intubations. In cases with a severe neck or oral contractions, the patient may be induced with an agent, such as ketamine, that will maintain spontaneous ventilation; following induction, the surgeon may release the contracture facilitating airway instrumentation. Since awake or moderate sedation intubation is not possible in children, ketamine seems the best choice for intubation in pediatric patients, since the pharyngeal tone is well maintained while also maintaining spontaneous breathing efforts.
24.4.2 Vascular Access
Vascular access in burned patients may be technically difficult due to large TBSA affected by the burn itself, peripheral edema resulting from massive fluid resuscitation, and multiple graft harvest sites occupying much of the unaffected skin. Both peripheral and central venous access, as well as arterial cannulation, may be achieved more safely and rapidly under ultrasonic guidance [53]. Although internal jugular cannulation is preferred for central venous access to minimize the risk of pneumothorax, alternate sites could include the subclavian or femoral veins. At times, it may be necessary to place catheters through burn wounds; in such cases, it is extremely important to meticulously prepare the area with antiseptic solutions just prior to placement of the catheter. Additionally, venous cannulation in burn patients comes with increased risks of bloodstream infection, as well as deep venous thrombosis due to prior venous cannulations , prolonged immobility, and hypercoagulability. Ultrasound guidance may help to diagnose the presence of an in situ clot, thereby avoiding futile attempts at cannulation at that site. Placement of central venous and arterial catheters under controlled conditions in the operating room is associated with a low rate of mechanical (0.3%) and thrombotic (0.6%) complications [53]. If no intravenous access can be obtained, patients of any age may receive a temporary intraosseous (IO) line until an alternative is available. Additionally, IO is our preferred method for emergent access should IV access be needed urgently or lost intraoperatively. One such scenario would be laryngospasm in a burned patient with no IV access.
24.4.3 Evaluation of Volume Status/Fluid Resuscitation
Prompt intravascular volume resuscitation is necessary to address acute shock in severe burn injury, in order to prevent hypovolemia and subsequent tissue hypoperfusion and multiple organ failure. Additionally, lung microvascular permeability changes seen with smoke inhalation are made worse by inadequate fluid resuscitation. However, overly aggressive fluid resuscitation may result in pulmonary, intestinal, and peripheral edema.
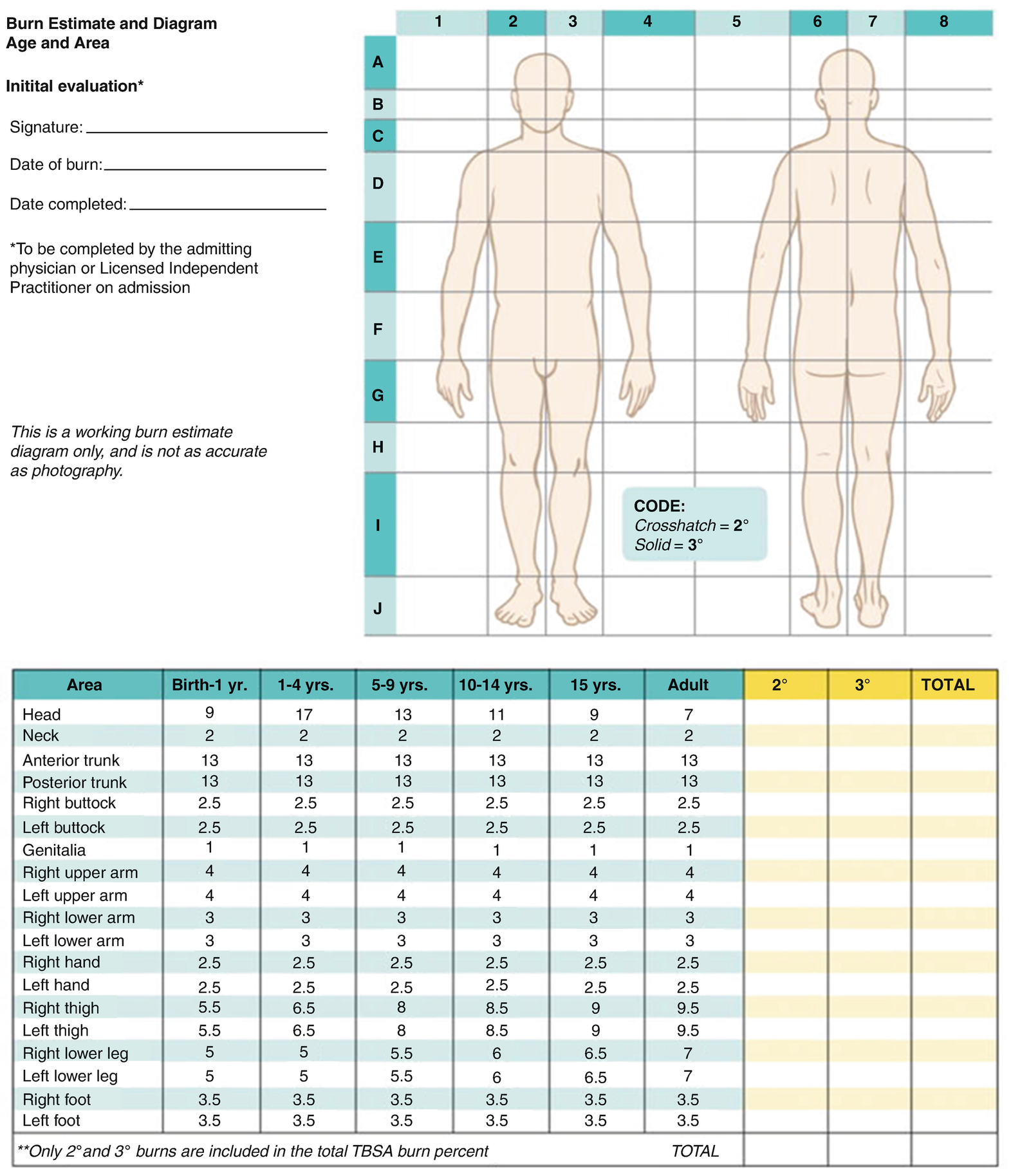
Lund–Browder burn diagram and table. The Lund–Browder burn diagram and table indicate the varying proportions in surface area across different ages. A diagram such as this one should be completed at the initial presentation to document the estimated size, location, and depth of a burn
Indicators of adequate fluid resuscitation
Parameter | Target |
|---|---|
Urine output | 0.5–1 mL/kg/h |
Blood pressure | Within normal limits (adjusted for age in children) |
Heart rate | Within normal limits (when pain and anxiety are adequately addressed) |
Central venous pressure | 3–8 mmHg |
Fractional excretion of sodium (FENa) | >1% (lower values suggest pre-renal injury and hypovolemia) |
BUN/Cr ratio | <20 (ratio >20 suggests maximal resorption of BUN and hypovolemia) |
Base deficit | <5 (larger values suggest hypoperfusion in the absence of carbon monoxide or cyanide poisoning) |
Conversely, high urine output has been seen in spite of hypovolemia, because of the osmotic effects of breakdown products and the increased GFR with tubular dysfunction. Often serum BUN to creatinine ratio of >20 indicates hypovolemia. In patients in whom these traditional measures are difficult to interpret, novel methods may aid in assessing fluid status. In addition to transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) [3], technetium-99m ventriculography has been used in burn patients to diagnose and treat low cardiac output states [56]. More recently, esophageal Doppler monitoring has been applied to burn shock resuscitation [57]. Noninvasive ultrasound-based cardiac output monitors (applied via a suprasternal or pulmonary window) have been piloted in other critically ill patients and may hold promise in burn patients, but their applicability will be limited in patients with burns affecting the chest and thorax [58].
Burns less than 15% TBSA may be treated with either oral or IV fluids administered at a rate 50% greater than the maintenance rate. For larger burns, the Parkland (Baxter) and Brooke formulae are the most commonly used guides. Both formulae may underestimate fluid needs in infants less than 10 kg. In these children, one may calculate the maintenance fluid requirements and add this to the amount suggested by either the Parkland or Brooke formulas, or one may modify the infusion volumes based on clinical responses.
When using crystalloid, often Lactated Ringer’s is chosen over normal saline given the hyperchloremic metabolic acidosis that may occur with massive administration of the latter solution. Alternatively, use of colloid may reduce the degree of peripheral edema that occurs with massive fluid resuscitation. Traditionally, the Parkland formula advocated a transition to colloid after the initial 24 h, but many burn centers have begun colloid administration earlier in burn wound resuscitation [59]. These may be particularly advantageous in very young children and in the elderly. While a recent Cochrane review found no difference in morbidity or mortality with the use of hypertonic saline versus isotonic saline, hypertonic saline did reduce overall fluid requirements, but with an accompanying transient increase in the serum sodium [60]. Currently most burn centers do not use hypertonic saline for resuscitation. After approximately 36–48 h, the permeability of the capillary wall returns to normal in non-burned areas, and peripheral edema begins to resolve over the subsequent 1–2 weeks. During this period, fluid requirements decrease and diuretics administered as needed to help mobilize the edema.
Severe burn patients are at increased risk for hyperosmolar, hyperglycemic non-ketotic coma, which is characterized by severe dehydration, marked hyperglycemia, and coma in the absence of ketoacidosis. Because of this, and in light of the insulin resistance and resultant hyperglycemia seen in acute burns, glucose-containing solutions are typically avoided, though they may be used in young infants and other patients at risk for hypoglycemia.
No clear, validated transfusion threshold exists across all burn patients. In each, it is most important to maintain adequate circulation and metabolic homeostasis. A national survey of burn centers revealed that the hemoglobin level below which clinicians would transfuse increases with increased % TBSA, history of cardiac disease, presence of ARDS, and age [61]. Blood products may be used in anticipation of continued blood loss, for example, during excision and grafting procedures. In addition to packed red blood cells, it may be appropriate to administer fresh frozen plasma in anticipation of the development of coagulopathy in patients undergoing massive blood loss intraoperatively, particularly when blood transfusion approximates one blood volume.
24.4.4 Temperature Regulation
The maintenance of normal body temperature is critical in both the operating room and the intensive care unit. Particularly susceptible periods include both the initial volume resuscitation and when dressings are removed for assessment and treatment. The initial inflammatory response to severe burns causes an increase in the hypothalamic core temperature set point, and hypermetabolism occurs (as above) to maintain this increased temperature. Hypothermia causes an increase in oxygen consumption due to shivering, which can exacerbate the catabolism seen with burn injuries. The shivering also causes dislodgement of grafted tissues. In addition, hypothermia during excisions can increase blood loss secondary to coagulopathy and has been shown to increase the risk of acute lung injury [62]. Maintenance of normothermia is made more difficult by the loss of the thermal regulatory function of intact skin. Efforts to maintain adequate body temperature are essential and may include increasing the ambient temperature (often as high as 80–100 °F), use of warming blankets and radiant warmers, administration of fluids and blood products via fluid warmer, minimization of exposed skin, and wrapping exposed skin in plastic insulation.
24.4.5 Pain Management and Opioid Sparing Techniques
Pain management in burn patients can be especially challenging, as nearly all aspects of burn treatment are associated with pain, including dressing changes, excision and grafting procedures, physical and occupational therapy, daily weighing, and vascular access procedures. In general, the severity of pain is proportional to the magnitude of TBSA burned, but it is important to recognize that psychosocial factors affect the individual’s experience of pain, as well. Poorly treated background pain can provoke anxiety which will exacerbate pain and lead to anticipatory procedural anxiety in a viscous cycle. Additionally, burn patients are shown to suffer from both hyperalgesia and allodynia.
Opioid administrations have been the cornerstone of pain therapy for many decades. While there has been significant fear of promoting long-term opioid addiction, treatment of adult burn patients with opioids has revealed a very low rate of addiction, and no reports of children developing addiction after therapeutic use of opioids for burn pain have been published. This research has led to some liberalization in opioid dosing to provide adequate analgesia in burn patients, but other concerns remain with respect to opioid use including opioid-induced hyperalgesia and immunosuppression . Patient-controlled analgesia (PCA) is one safe and effective mode of delivering opioids in burn injuries [63]. The presence of bandages on the hand may preclude the use of PCA. It is important to recognize that opioid tolerance will develop and appropriate dose adjustments made. Opioid requirements tend to decrease dramatically following successful closure of the thermal wounds. The most effective analgesic and anxiolytic strategy is to ensure that definitive wound closure happens as expeditiously as possible. Counter to the acute effects of sedatives, which potentiate the effects of opiates, a rodent study suggests that prolonged administration of midazolam with morphine accelerates the development of hyperalgesia [64].
Alternative or adjunct analgesics include ketamine, dexmedetomidine, gabapentin, and acetaminophen and methadone. Ketamine may have particular utility in counteracting the hyperalgesic effects of upregulated N-methyl-d-aspartate (NMDA) receptors seen after burn; it may be used as an adjunct analgesic intraoperatively, as a continuous infusion in the intensive care unit, or bolused for painful bedside procedures, such as dressing changes. Ketamine may also possess anti-inflammatory effects in patients with burns and/or sepsis [65] and mitigate opioid-induced hyperalgesia [66]. Dexmedetomidine is a parentally administered α2-agonist with sedative, anxiolytic, and analgesic effects. It has been demonstrated to reduce opioid requirements post-operatively in adults [67], but while it appears to provide good sedation for pediatric burn patients, it consistently decreases mean arterial pressure and may not diminish opioid requirements in children particularly with long-term use due to the development of tolerance to dexmedetomidine [68]. Gabapentin reduces opioid consumption and lowers pain scores, with these effects extending beyond the duration of pharmacologic action, indicating a likely role in the mitigation of opioid-induced hyperalgesia [69]. While acetaminophen has a ceiling effect and is generally insufficient to adequately control burn-related pain on its own, it has proven opioid-sparing effects and should be considered in conjunction with the analgesics described above. Because of the potential for bleeding, NSAIDs (including ketorolac) are generally avoided in the acute phase of burn. Methadone, in addition to being an opioid. has multiple other sites of action enhancing analgesia. Its main downside is the variable half-life influenced by genetic and co-administered drugs [70].
Finally, the potential for a regional anesthetic should be considered in all burn cases, especially when adequate analgesia is expected to be a challenge. The benefits of regional anesthesia include not only superior intraoperative and postoperative analgesia but also facilitation of earlier rehabilitation via participation in physical and occupational therapies. Pain from a split-thickness donor site often exceeds that from the actual grafted burn wound. In cases where the size of the donor site is not excessive, the surgeon may inject tumescent local anesthesia into the donor site prior to harvesting; the size of site that may be covered with such a technique is limited by the accepted maximal local anesthetic dose (e.g., lidocaine 7 mg/kg or bupivacaine 2.5 mg/kg maximum) [71]. The placement of subcutaneous catheters to the donor sites has also been described and provided postoperative analgesia for a mean of 3.1 days [72]. Traditional peripheral nerve blocks, with or without continuous infusions via a catheter, improve postoperative pain control versus local anesthetic infiltration alone [73]. Neuraxial techniques (i.e., spinals and epidurals) may also be used with good effect, as well as truncal blocks, such as paravertebral and transversus abdominis place (TAP). The lateral femoral cutaneous block is particularly useful as the lateral thigh is frequently chosen as a donor site for split-thickness skin graft, and the block offers the advantage of being purely sensory. In cases where coverage of the anterior and medial thigh is also desired, the lateral femoral cutaneous block may be combined with a fascia iliaca block [74].
24.5 Conclusion
The perioperative care of burn patients involves complex pathophysiologic changes, which evolve throughout the course of injury and recovery. The anesthesiologist must face these challenges, anticipate alterations in pharmacodynamics and pharmacokinetics, and address the procedural complexities of airway management and obtaining venous access. Pediatric and geriatric patients require special consideration, as well. New advances in regional anesthesia/analgesia and multimodal therapies are allowing for opioid sparing while optimizing pain control. These factors are best addressed through a multidisciplinary collaboration, so that the patient may be cared for in a streamlined, coordinated fashion throughout the perioperative period.
Summary Box
Anesthesiologists often play a critical role in the initial stabilization of acute burn patients, as well as in the perioperative care of both acute and chronic burn injuries. Thus, they must understand the pathophysiological impact of large total body surface area burns on each system and adjust their anesthetic management accordingly. Both pediatric and geriatric patients are at increased risk of burn injury and merit special attention to psychologic and comorbid medical conditions. Appropriate anesthetic and analgesic medication dosing will require consideration of the stage of burn injury in order to accommodate for changes in pharmacokinetics. Tolerance is also a frequent problem with severe burn injury, prompting the anesthesiologist to pursue alternative pain and sedation regimens, incorporating multimodal and regional analgesia techniques. Finally, anesthesia providers must be prepared to address the specific challenges to airway management and vascular access posed by severe burn injuries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







