Aminoquinolines: Introduction
|
Antimalarial compounds have been used to treat skin diseases for more than a century. Three compounds, chloroquine (CQ), hydroxychloroquine (HCQ), and quinacrine (QE), have been most studied. The 4-aminoquinolines are a family of compounds derived from quinine, a naturally occurring alkaloid originally procured from the South American cinchona bark tree.1 CQ and HCQ, the principal members of this class of compounds, are the most often used for therapeutic use in dermatology. QE, classified as an acridine, has also been used in dermatologic therapy. Interestingly, the use of these medications for treatment and prophylaxis of malaria is waning due to increased resistance.
Mechanism of Action
The precise mechanisms by which antimalarial compounds exert their actions are still unknown. However, several discrete mechanisms for each compound have been determined. In the treatment of malaria, aminoquinolines concentrate in the Plasmodium digestive vacuole and prevent polymerization of toxic heme released during proteolysis of hemoglobin.2 Mechanisms by which these compounds influence skin diseases are varied and include, among others, their effects on antigen presentation, cytokines, toll-like receptors, and prostaglandins.
CQ and HCQ are lipophilic weak bases that pass through plasma membranes in their protonated state, become trapped, and accumulate inside acidic vesicles such as lysosomes,1,3,4 accounting for their effects in Plasmodium. HCQ induces signs of lysosomal and mitochondrial membrane permeabilization.5 By raising intralysosomal pH from 4–6, acidic proteases become inactivated and subsequent proteolysis is inhibited. Subsequently, antigen processing and presentation by dendritic cells may be impaired because of an inability to digest these foreign antigens within the lysosomal compartment.6 Additionally, CQ has been shown to disrupt antigenic peptide loading on class II MHC molecules and subsequent presentation to the opposing T cell.1,7 Inhibition of calcium signaling within the T cell may be an additional mechanism.8 Conversely, CQ has been more recently shown to enhance human CD8+ T cell responses.9 QE inhibits Na-K adenosine triphosphatase activity and stabilizes cell membranes.10 It also concentrates in lysosomes. The effects are most appreciated in macrophages and phagocytic cells where phagocytosis and chemotaxis are impaired.11,12 Additionally, cellular enzymes and receptors dependent on lysosomes for function or activation are also affected, thus altering cells’ responsiveness to mitogenic stimuli.3
Antimalarials decrease T-cell release of interleukin (IL)-1, IL-6, tumor necrosis factor-α, and interferon-γ.13,14 IL-1, IL-6, and tumor necrosis factor-α play a role in the hepatic production of acute-phase reactants.15 Low concentrations of CQ and QE inhibit the pro-IL-6 stimulatory effect of cytosine-phosphorothiolated guanine-oligodeoxynucleotides on human peripheral blood mononuclear cells.16,17 More recently, it has been found that CQ elevated the expression of B7–2 (CD86, a costimulatory molecule) and intercellular adhesion molecule-1 (CD54, an adhesion molecule) in peripheral mononuclear cells, thus increasing IL-10-producing cells.18 CQ has also been implicated in the inhibition of natural killer cell activity, decreased production of IL-2 from activated lymphocytes, and alteration of antigen-antibody complexes. QE also inhibits natural killer cells and blocks the primary proliferative response of cytotoxic T cells to allogeneic non-T-cell antigens.15 Last, the arachidonic acid pathway is affected by antimalarial compounds. CQ inhibits phospholipase A2 and C.15 QE also inhibits phospholipase A2 production19,20 and increases nitric oxide release.21 Thus prostaglandin, leukotriene, bradykinin, and histamine levels can all be decreased. QE antagonizes the generation of superoxide anions.22
A novel mechanism of action of the antimalarials has recently been elucidated with respect to its inhibition of toll-like receptor (TLR) 9 family receptors.1 TLRs are intracytoplasmic receptors whose activation of the innate immune system in response to microbial peptides induces a significant inflammatory response. Additionally, further research has highlighted the importance of the innate immune response underlying the pathogenesis of systemic lupus erythematosus (SLE).23 Consequently, these TLRs who, as a class, are known as “pattern recognition receptors” (PRRs), have become a subject of intense investigation underlying the pathogenesis of SLE. It has recently been shown that host immune complexes containing DNA or RNA play an important role in activating endogenous TLRs (especially TLR-9 and TLR-7), thus leading to the eventual activation of the innate immune system, of which interferon alpha plays a crucial role.24,25 Additionally, TLR-9 expression is strongly associated with SLE activity.25 These specific nucleic acid binding TLRs bind their ligands in the lysosome, where an acidic environment promotes this binding.26 As the antimalarial agents specifically target microsomes by disrupting endosomal maturation and changing the pH, they block the TLR interaction (TLR-3, -7, and -9) with nucleic acid ligands.27 In vitro studies have identified that nanomolar concentrations of chloroquine specifically were potent inhibitors of IL-6 production by monocytes, an effect now known to be directly mediated by the inhibition of TLR-9.1 Antigen presenting cells are the primary targets of these interactions, thus accounting for a clinically slower response, as they initiate and prime the subsequent immune reactions.1 The 4-aminoquinolines have an affinity for melanin pigment. In the epidermis (as well as retina), they bind in high concentrations. In the skin, CQ absorbs ultraviolet (UV) light in a concentration-dependent manner.28 There is an increase in minimal erythema doses (MEDs) to UVB in lupus patients after taking oral chloroquine for 3 months, theoretically resulting from the anti-inflammatory and/or photoprotective mechanisms of the drugs.1,29 Topical CQ applied before UV irradiation protects against UVB- and UVA-induced erythema.30 QE has also been shown to inhibit photodynamic actions.31 It has been proposed that the beneficial effect of the HCQ and CQ in various photodermatoses may result from the ability of these drugs to enhance the protective early limb of the UV response.32
CQ and QE have been shown to bind cellular DNA by intercalation between base pairs, thus stabilizing the DNA.33–35 RNA transcription and subsequent translation are inhibited. These may be the mechanisms by which these molecules affect lupus.36
Antimalarial compounds also exhibit a myriad of ancillary effects. HCQ has been shown to improve lipid profiles, specifically causing a 15%–20% reduction in serum total cholesterol, triglycerides, and LDL levels.37 Both CQ and HCQ improve glucose profiles by decreasing insulin degradation38,39 and inhibit platelet aggregation.40 High doses of CQ may produce an antioxidant effect.41 Through membrane stabilization, antimalarials can produce a local anesthetic effect.3 In vitro, HCQ has been shown to induce apoptosis in B-chronic lymphocytic leukemia cells.42 CQ, HCQ, and QE have all been shown to interfere with human immunodeficiency virus type 1, SARS coronavirus, and influenza replication and function possibly due to disruption of protein glycosylation.43–46
Pharmacokinetics
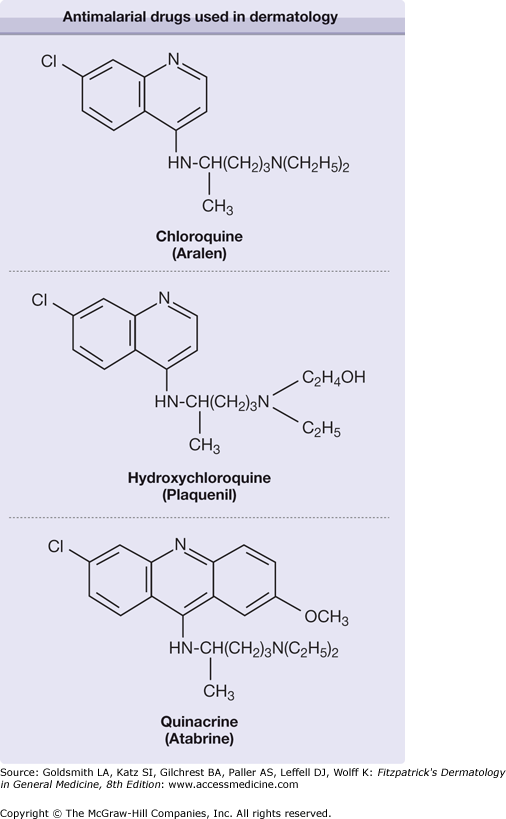
The molecular structures of CQ, HCQ, and QE are closely related (Fig. 226-1). CQ and HCQ are 4-aminoquinolines, with HCQ differing from CQ by the presence of a hydroxyl group at the end of the side chain.1 The addition of a benzene ring classifies QE as an acridine. The pharmacokinetics and pharmacology of these compounds are similar, but importantly there is no cross reactivity between the aminoquinolines and QE (Table 226-1). All are bitter, water-soluble, and readily absorbed from the gastrointestinal tract with relatively good bioavailability, achieving peak concentrations within 8–12 hours. CQ and HCQ have large volumes of distribution (over 100 L/kg) and prolonged half-lives.47 The volume of distribution for QE may be even larger than that of CQ and HCQ. QE is extensively bound to plasma proteins, whereas CQ and HCQ are only partially bound (60%).48–50 The half-life of QE is substantially less than the other compounds.50 CQ is metabolized (dealkylated) in the liver by P450 enzymes into two active metabolites, desethylchloroquine and bisdesethylchloroquine.51 The metabolic fate of QE is less certain. CQ and its metabolites are stored in relatively high concentrations in the liver, spleen, lungs, adrenals, and kidneys with the highest concentration found in melanin-containing cells of the retina and skin.52 Approximately 50% of CQ is eliminated by the kidneys unchanged.48 HCQ is also metabolized in the liver (N-desethylated) into three chief metabolites: (1) desethylhydroxychloroquine, (2) desethylchloroquine, and (3) bisdesethylchloroquine.47 The parent compound and metabolites are taken up by lysosomes and concentrated in lysosome-rich tissues. Renal clearance accounts for approximately 15%–25% of the total clearance.53 The renal excretion of CQ, HCQ, and QE can be enhanced with acidification of the urine.54 QE is excreted in urine, bile, sweat, and saliva.50 Renal excretion only accounts for approximately 11% of its clearance. The highest tissue concentrations of QE are found in the liver and spleen.52 Equivalent doses of these compounds are 250 mg of CQ, 400 mg of HCQ, and 100 mg of QE. Smoking may alter the pharmacokinetics of antimalarial compounds, as clinical evidence of decreased efficacy of these medications in smokers has been documented.55 Decreased absorption, increased plasma clearance, or induction of the cytochrome P450 system may be mechanisms by which smoking alters the metabolism of these compounds.56
Chloroquine (Aralen) | Hydroxychloroquine (Plaquenil) | Quinacrine (Atabrine) | |
|---|---|---|---|
Bioavailability | 80%–90% | 75%–100% | 80%–100% |
Peak levels | 4–8 hours | ∼4 hours | 8–12 hours |
Protein binding | 50%–65% | 45%–50% | 80%–90% |
Volume of distribution | 13,000–65,000 L | 5,500–43,000 L | — |
Clearance | 130 mL/min (blood) 1,100 mL/min (plasma) | 95 mL/min (blood) 700 mL/min (plasma) | — — |
Half-life | 20–60 days | 40–50 days | 5–14 days |
Metabolism | Dealkylated (liver) | Deethylated (liver) | — |
Metabolites | Desethylchloroquine Bisdesethylchloroquine | Desethylhydroxychloroquine Desethylchloroquine | None |
Renal elimination | 50% | 15%–25% | 11% |
Storage | Liver, spleen, lungs, adrenals, pigmented tissues | Adrenals, pituitary, liver, spleen, leukocytes, pigmented tissues | Liver, spleen, lung, adrenals |
Equivalent doses | 250 mg | 400 mg | 100 mg |
A recent study has highlighted the relationship between whole blood concentrations of HCQ and disease activity in SLE patients.57 Results of whole blood levels in 143 SLE patients who had received 6 months of HCQ at a dose of 400 mg/day demonstrated large variability in blood concentrations (possibly due to compliance). The mean concentration of patients with inactive disease was 1,079 ng/mL compared with the mean level of patients with active disease, which was 694 ng/mL. Additionally, the authors found that a low blood level of HCQ was associated with disease exacerbation in the follow-up period. The conclusion was that whole drug concentrations of HCQ should be maintained above 1,000 ng/mL, indicating a need for regular drug assaying and individualized dosing schedules to maintain this level.
Typical doses used in dermatology are 250–500 mg/day for CQ, 200–400 mg/day for HCQ, and 100–200 mg/day for QE. Evidence of efficacy is generally seen after 1–3 months of use, but it may take up to 3–6 months to achieve maximum clinical efficacy. After this time, the dose may be tapered to a minimum effective dose. Again, there is no cross-reactivity between QE and CQ and HCQ.58 Thus, the use of QE in combination with HCQ and/or CQ has been deemed safe, and an adverse reaction to the 4-aminoquinolone group does not preclude the use of QE. In refractory skin conditions, addition of QE to HCQ or CQ has been deemed beneficial, and response may be seen in 6–8 weeks after the addition of QE.59 If QE is added to CQ, the dose of CQ may need to be decreased to reduce potential for toxicity. Triquin, a combination of CQ (65 mg), HCQ (50 mg), and QE (25 mg) was used in the early 1970s. Nevertheless, the concomitant use of CQ and HCQ increases the risk for retinopathy (see Section “Complications”). Thus, this combination should be restricted to only severe cases. QE is not currently commercially available; production stopped in 1992. QE powder (veterinary grade, Sigma) can be formulated into capsules. Panorama Pharmacy in California, with an additional purification step, will prepare QE capsules.
Indications
The US Food and Drug Administration-approved indications for CQ, HCQ, and QE are limited. As is often seen in dermatology, these compounds are frequently used in an off-label manner. Treatment of malaria is the only indication shared by all three drugs. HCQ has the most labeled uses, including rheumatoid arthritis, discoid lupus, and systemic lupus erythematosus. Extraintestinal amebiasis is the only other approved use for CQ. QE had been approved for treatment of giardiasis when it was manufactured. Experience with HCQ in dermatology is more extensive. Its lower incidence of ocular toxicity (see Section “Complications”) makes it a favored choice. In recalcitrant disease, CQ is often substituted with increased efficacy. The current difficulty in obtaining QE may account for the relatively fewer indications (Table 226-2).
Hydroxychloroquine (Plaquenil) | Chloroquine (Aralen) | Quinacrine (Atabrine) |
|---|---|---|
|
|
|
(See Chapter 155)
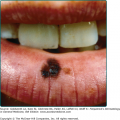
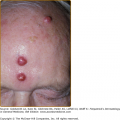
Antimalarial drugs have been used for the cutaneous manifestations of lupus (acute cutaneous, subacute cutaneous, and chronic cutaneous LE) since the 1930s.60 CQ and QE were found to be effective in the treatment of cutaneous lupus also during World War II.61 A double-blind trial of HCQ for discoid lupus showed a 70% response rate and that it was more efficacious than placebo at 3 months and 1 year of treatment.62 A review of 20 clinical trials involving the use of QE for cutaneous lupus showed an overall 73% response rate.63 Antimalarials are typically used as steroid-sparing agents in patients whose symptoms cannot be controlled by topical steroids and sun protection. Widespread cutaneous lesions, hypertrophic and verrucous lesions, and longstanding discoid lesions do not respond as well. Systemic symptoms such as fever, renal, or hematologic abnormalities seen in systemic lupus erythematosus typically do not respond to antimalarial therapy, but fatigue, arthralgias, myalgias, serositis, and mucosal ulcerations do respond to therapy.64 In addition, antimalarials have effectively treated nonspecific cutaneous manifestations of LE, such as mucosal ulcerations, calcinosis cutis, lupus panniculitis, and photosensitivity.65 Lupus panniculitis specifically has been reported to respond well to antimalarial therapy, with 23 out of 33 patients in one study demonstrating clinical improvement.66 The onset of action for antimalarial therapy is typically 4–8 weeks; after which time, one may consider adding or changing therapies. More rapid response may be seen with the addition of QE.63 When HCQ is ineffective as a monotherapy, CQ may be more efficacious, but the combination of HCQ and CQ is generally avoided to prevent retinal toxicity. The use of QE alone or in combination with either HCQ or CQ has also been suggested as providing a response in recalcitrant patients. A recent report evaluating 34 patients unresponsive to HCQ therapy alone identified the majority of patients rapidly and significantly responded to the addition of QE to their therapeutic regimen.67 Triquin, a combination of all three drugs, has also been used in cutaneous lupus erythematosus with success.
(See Chapter 91)
Polymorphous light eruption (PMLE) is an inflammatory reaction seen on sun-exposed skin. IL-1, -6, and -8 may play a role in the inflammatory process.68 Chloroquines decrease IL-1 and -6 and release and absorb UVA light. Although generally not used as first-line treatments, these drugs have been used for PMLE for several years69,70 and placebo-controlled trials have proven efficacy for this indication.71 Two trials demonstrated increased sun tolerance and moderate clinical improvement identified by skin rash reduction, which was statistically significant.1,72,73 Intermittent HCQ at doses of 200–400 mg/day prior to sun exposure can be effectively tried if photoavoidance and/or prophylactic UVB/UVA are not viable options. Topical sunscreens should also be used in conjunction with antimalarial therapy. In northern climates, discontinuation of therapy during winter months may decrease cumulative side effects of antimalarial therapy.
(See Chapter 132)
The first reported case of CQ therapy for porphyria cutanea tarda (PCT) was published in 1957,74 and its use can lead to long-term remission.1,75 Chloroquines inhibit porphyrin synthesis and mobilize lysosomal stores of porphyrins, allowing for the formation of water-soluble complexes, which are then excreted by the kidneys.76 Although phlebotomy remains the primary treatment of choice for PCT, antimalarial therapy may be used in conjunction with phlebotomy or when phlebotomy is contraindicated or has previously been used without success. Hepatotoxicity is a concern, and liver enzymes should be followed closely during therapy. Headache, nausea, fever, elevated transaminases, and urinary excretion of porphyrins occur 3–4 days after initiation of therapy with CQ at 250 mg/day.77 Prolonged remissions have been reported with short courses of high dose HCQ (250 mg three times daily), but high doses routinely lead to marked increases in liver transaminases.78 Low-dose CQ therapy (125 mg twice weekly for 2 weeks followed by 250 mg twice weekly thereafter) gave a 94% response rate in one study of 53 patients.79 Alternatively, HCQ used at 100 mg three times weekly for 1 month, then 200 mg three times weekly for 1 month, then advanced to 200 mg daily has been proposed.80 These lower dosing schedules seem to alleviate the severe acute hepatotoxicity. A combination of phlebotomy before initiation of CQ therapy may also reduce the severity of hepatotoxicity.81
(See Chapter 152)
Antimalarial therapy for cutaneous manifestation of sarcoidosis was first described nearly half a century ago. Original reports on the efficacy of antimalarials in the treatment of sarcoidosis demonstrated initial clinical improvements on treatment during a 6-month course, with little further improvement after 1 year, indicating the treatment was likely suppressive rather than curative.82 Additionally, relapses off of these medications are common, further supporting the notion that these therapies are not curative.82–87 The proposed mechanism of action relies on the antimalarials’ capacity to block antigen processing and presentation to CD-4+ T cells, thus inhibiting granuloma formation.83,88 A 1996 review of the subject concluded that antimalarials were highly effective, with greater than 70% response rate.89 Two to three mg/kg/day of HCQ has been reported as being effective in 12 of 17 patients treated.90 CQ therapy initiated at 500 mg/day for 2 weeks, then a maintenance dose of 250 mg/day has been described as safe and effective.91 Therapy may also be effective for associated hypercalcemia. Another granulomatous disease, generalized granuloma annulare, has also been reported to respond to HCQ.1,92–94
(See Chapter 156)
Although the muscle disease in this condition often responds well to systemic corticosteroids, the cutaneous lesions are generally recalcitrant to this therapy. Cutaneous symptoms unresponsive to traditional therapies have been reported to respond well to antimalarial therapy. It is in these patients that antimalarial therapy be strongly considered, as well as in those patients with cutaneous involvement without any muscle involvement (“amyopathic dermatomyositis”).1 Monotherapy with either HCQ 200 mg bid (less than 6.5 mg/kg/day) or CQ 250 mg qd (less than 3.5 mg/kg/day) is often adequate to control skin manifestations. The addition of QE to either HCQ or CQ has been shown to be beneficial in patients who do not respond to a 4-aminoquinolone alone.59
Because the lesions of oral LP are clinically indistinct from those of mucosal LE, an open trial enrolling oral LP patients was undertaken to assess their response to HQC.95 Nine out of the ten enrollees exhibited a rapid, excellent response to therapy with 200–400 mg/day HQC, with a minority flaring after discontinuation of therapy (3 months). Although corticosteroids are the first-line therapy for this disorder, recalcitrant lesions may respond to antimalarial therapy.
There are only a handful of cases reported in the literature describing this rare disorder which occurs primarily in older female patients. Clinicopathologically, this disease mimics erosive lichen planus but is traditionally unresponsive to corticosteroid therapy. The cases reported, however, demonstrated a complete response to antimalarial therapy lasting for several months to years. These medications are now considered first-line therapy for this rare condition.96