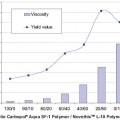
Fig. 1
This can be demonstrated in the following table:
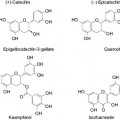
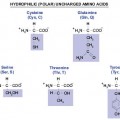
Structures of Amino Acids (Table 1)
Solubility curves of amino acids that are relatively soluble in water

Figure 2
The solubility of L-Alpha Amino Acids is shown in the following charts:
The “breaks” in the curves of the behavior of some of the amino acids shown are the temperatures at which one mole of bound water is given up by that amino acid.
Solubility curves of amino acids that are relatively insoluble in water
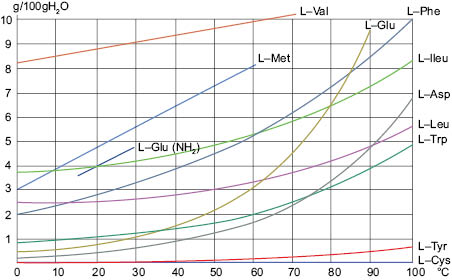
Figure 3
It has been found that topically applied amino acids absorb into the epidermis and into the hair shaft. The first was done by a skin-stripping study and the latter through radio-tagged amino acids and amino acid analyzer.8
The amino acid analyzer measures the amino acids that are added to the hair shaft as well as those that are washed out. The radio tagging only measures amino acids that are added to the hair shaft. By using a combination of these analyses it is possible to determine how much amino acid penetrates the hair.
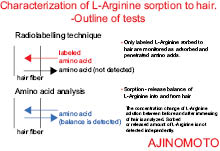
Figure 4
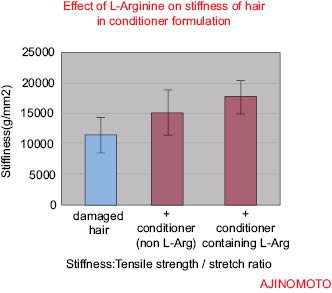
Figure 5
Function in Body Table 2: Amino acids as the building blocks of the body have many positive effects.
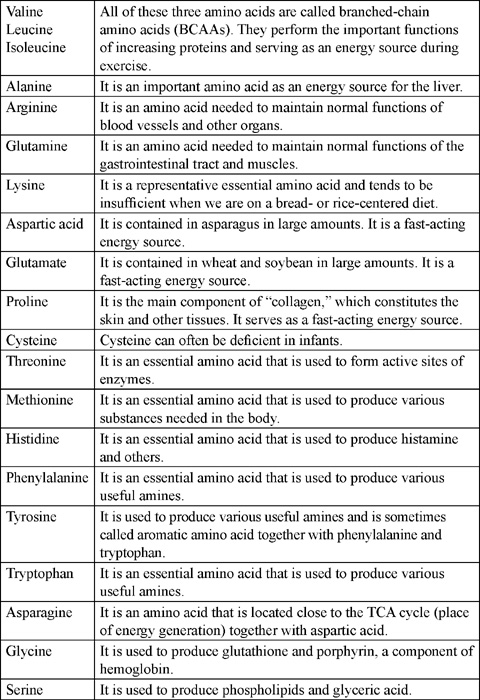
1.1 Derivatives of Amino Acids for Personal Care formulation:
Amino acids can be derivatized into many useful materials for formulation in the cosmetic and personal care field. The following chart shows just a few of the possibilities. Glutamic acid can be acylated with a fatty acid and neutralized to produce an anionic surfactant acyl glutamate. Glutamic acid can also be acylated and esterified to produce emollients, and it can be cyclized by dehydration to form a humectant. Aspartic acid can be polymerized and neutralized to produce polyaspartate, a film former and humectant. Latter sections in the chapter will discuss the use and benefits of many of these derivatives.
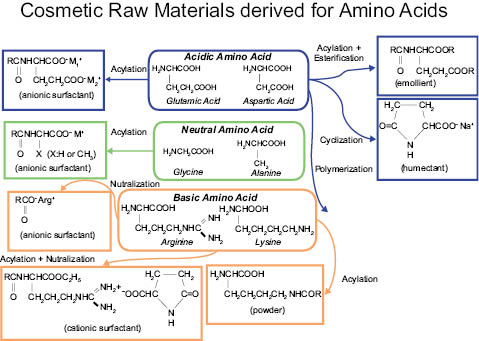
Fig. 6
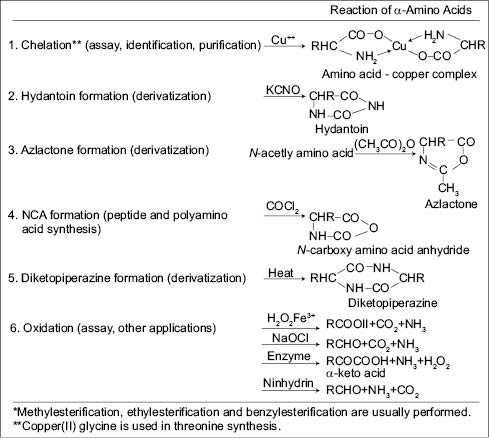
The following are some of the reactions that are possible with amino acids:
Table 3
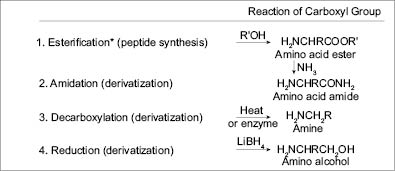
Table 4
As formulators it is important to keep these possible reactions in mind.
4.3.4.2 THE APPEARANCE OF AGING OF SKIN AND HAIR
We all see the signs of aging. Skin that is wrinkled, dry, and fragile. Hair that breaks easily, is dull and thinning. Sometimes these signs appear with the accumulation of years. Sometimes they appear as a result of disease, or of abuse, such as unprotected chronic tanning. Medical conditions must, of course be addressed by the appropriate physician. The personal care formulator can be and is equipped to formulate products that mitigate the appearance of aging and in some cases (e.g. the use of sunscreens) can prevent, the damage to a large extent before it occurs.
What are the antagonists of skin and hair?
Free radicals top the list. Free radicals are produced in all cells and in macrophages in large amounts. They support the immune system and are important for health. However, an excess of free radicals can attack healthy cells and tissue. Free radicals are highly reactive molecules that can damage cell membranes, lipids, proteins, and DNA.10 Species such as singlet oxygen, superoxide, lipid peroxides, hydrogen peroxide, and hydroxyl radicals can and do have significant effects on the skin and the hair. Formulations that counter the buildup of reactive oxygen species (ROS) go a long way towards mitigating the appearance of aging.
The skin is the largest organ of the body and is responsible for protecting it from environmental impacts. At the same time the skin sends a signal regarding the health and attractiveness of the person. Sagging skin, flaking dry skin, loss of elasticity, dullness—these are some of the signs of aged skin and are seen as unattractive.
The signs of aged skin can be caused by a number of things. These include, but are not limited to the accumulation of years, environmental damage, illness, poor diet, lack of exercise, and lack of sleep. Personal Care/Cosmetic Science can address these issues. Of course in the case of illness and disease the root cause must only be addressed by the proper medical professional, but even in this case personal care/cosmetic science can help the patient look and feel better while they are undergoing treatment and also afterwards. A good example of this is the American Cancer Society’s “Look Good, Feel Better” program on how personal care/cosmetic science can help patients undergoing treatment.
There is significant evidence that topically applied amino acids penetrate into the epidermis. This was demonstrated by Maibach, et al.,11 Coderch et al.,12 Ruland et al.,13 and others. But what effect do they have on the appearance of the skin?
The effect of absorbed amino acids into the skin results in a moisturiziation of the stratum corneum, thereby making the skin smoother and more supple.
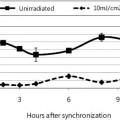
It has also been shown that in the case of atopic dermatitis (dry, flaky, itchy skin), the amount of every standard amino acid normally present in the skin is significantly reduced, as is the total amount of these amino acids—which can be seen from the following graph.
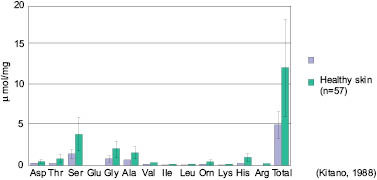
Figure 7
The mechanism for this aspect of atopic dermatitis may be the decrease in amino acid metabolic conversion into metabolites in hyperkeratosis. Some of the metabolic pathways may be shown as in the following table.
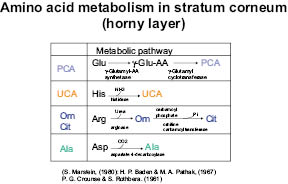
Figure 8
The rate of conversion to metabolites is greatly decreased in hyperkeratosis skin.
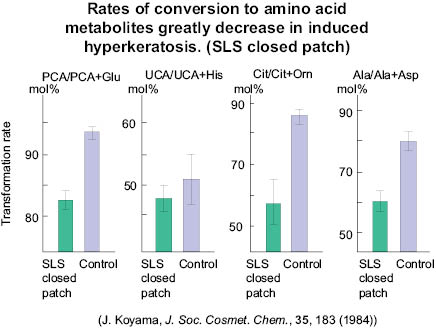
Figure 9
The topical application of amino acids to the skin helps to mitigate the dryness, flaking, and itching of the skin and restores a more natural and healthy amino acid balance, again making the skin appear more youthful.
Of all the things that adversely affect the skin and cause the appearance of aging under “normal” circumstances, free radicals and reactive oxygen species are arguably the worst offenders. Free radicals and reactive oxygen species (ROS) induced by environmental causes and UV radiation can overwhelm the skin’s natural antioxidant defenses. This can lead to wrinkling, collagen breakdown, the loss of elasticity, and a myriad of diseases associated with oxidative stress.14 It has been shown that topically applied antioxidants have a positive effect on the appearance and health of the skin.15
The work of Podda et al. was focused on vitamin C, vitamin E, ascorbic acid, tocopherol, and lipoic acid and showed that these small-sized antioxidants penetrated the skin with a positive effect. Amino acids are similarly small molecules and have indeed been shown to penetrate the skin. Cysteine (and cystine, since the S-S bond is easily broken), methionine, taurine, and N-acetyl cysteine are powerful antioxidants that will penetrate the skin. The tape-stripping work of Coderch et al. on threonine, isoleucine, and lysine shows that amino acids penetrate to 16 strippings, while Kawasaki, Sakamoto, and Maibach, working with glycine, sodium glutamate, proline, and threonine, show that there is penetration to 10 strippings.
Additionally, the prevention of ultraviolet light-induced wrinkles may be accomplished by the application of zinc pyrrolidone carboxylate prior to UV exposure.
Zinc PCA does this by interfering with the production of activator protein -1, AP-1. UV radiation initiates the formation of AP-1, which induces the production of collagenase. Collagenase causes the degradation of collagen, which then causes a wrinkle. If the cycle is broken, that wrinkle formation is stopped to a good extent. It has been shown in in vitro testing on human fibroblasts that 3 ppm zinc PCA inhibited 69.3% of AP-1 production and that 30 ppm of zinc PCA inhibited 77% of AP-1 production. It is expected that in in vivo work, somewhat more of zinc PCA would be required to produce the same effects.
Another cause of wrinkles is a lower rate of collagen production. If collagen production slows, the appearance of wrinkles increases. It has been shown that the topical application of an amino acid cocktail featuring a 1:1:1 molar ration of glycine: proline: alanine doubles the rate of collagen production. A 3:2:1 molar ratio of those same amino acids increases collagen production fourfold.16 This goes a long way to reduce the appearance of wrinkles and is noticeable for as long as the application of the amino acid mixture is used.
Lysine has been reported to have a positive cosmetic effect on wrinkle formation. It is known that lysine is needed for the production of collagen and that lysine-poor diets may increase the development of wrinkles. The thought is that since nutritional lysine supplementation has a positive effect on collagen production and it has been shown that topically applied amino acids also have a positive effect, then lysine topically applied may also help with the reduction of wrinkles. Other amino acids that have an effect on skin wrinkling are creatine, carnitine, arginine, and methionine. In these cases it has been reported that these amino acids stimulate regeneration of connective tissue.
Fine lines may be temporarily removed by the application of highly hygroscopic materials that supply moisture to the stratum corneum. Sodium PCA and proline are two candidates that have been shown to moisturize skin very efficiently. This moisturization “plumps” the skin slightly, removing the fine lines to a greater or lesser extent. Additionally, amino acid derivatives such as phytosterol/decyltretradecyl myristoyl methyl meta-alaninate binds in the order of 25 times its own weight in water, which provides a reservoir for the skin to use. Other analogs of this material such as phytosterol/behenyl/octyldodecyl lauroyl glutmate hold six times their own weight in water.
The topical application of amino acids and derivatives can increase the elasticity of the skin. Pig skin treated with sodium PCA has been tested using a Fudo rheometer. In this case, after treatment with a humectant, the rheometer measures the millimeters of skin elongation until it breaks.
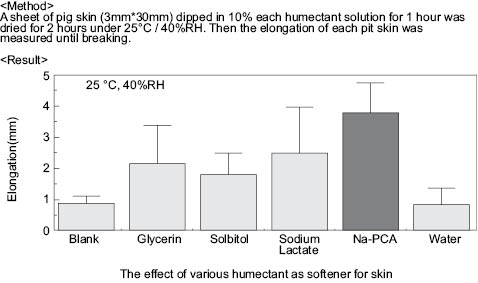
Figure 10
Sodium PCA increases the elongation of the skin dramatically over that of untreated and just water-treated skin. It also shows better results than other common humectants. Similarly, amino acid cocktails comprised of sodium PCA, betaine, serine, glycine, glutamic acid, alanine, threonine, and proline have similar elongation effects.
A demonstration of the effects of sodium PCA treatment on the skin is as follows:
Sodium polyaspartate treatment increases elasticity a good deal. A 10% active ingredient treatment of sodium polyaspartate allows the skin to stretch 5.75 mm, while a 10% Active Ingredient treatment of hydrolyzed animal collage permits the skin the stretch 1.5 mm, and no treatment 1.25 mm. The measurements are done on a Fudo rheometer at 25°C and 40% RH. In this test a specimen is placed between two grips and the instrument stretches the specimen at constant rate until breaking. The length of the specimen at breaking is recorded and sometimes the breaking force is also recorded. This instrument is similar to a universal testing machine used in tensile strength measurements.
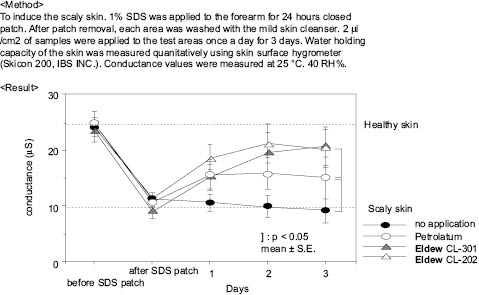
Figure 11
The use of alpha hydroxy acids and beta hydroxyl acids has become almost commonplace to clarify the skin. In clarifying the skin the dead and dull skin cells at the surface are removed; a process that allows the healthy and “bright” skin cells to be seen. These materials are very good keratolitics that consume the dead surface skin cells, allowing the skin to look fresh and clear. A keratolitic is a substance that softens the skins keratin and allows the dead dull skin cells to fall off or be easily removed. One difficulty with alpha or beta hydroxyl acids is that these materials are very acidic and produce a stinging sensation. Their stinging can be reduced by adjusting the pH, typically form 3 to a pH of 4.5. However, the use of normal neutralizing agents such as NaOH of fatty amines to bring the pH to 4.5 will also reduce the clarifying effect of the alpha and beta hydroxy acids. The amino acid arginine, an alkaline amino acid, has been shown to have the ability to raise the pH of the alpha or beta hydroxy acid product without reducing its efficacy.17, 18
Hydration of the skin is a key to a youthful appearance.
It has been shown that there is a linear correlation between the amount of amino acids present in the stratum corneum (SC) and the degree to which the skin is hydrated. The greater the amount of amino acids present, the greater the hydration.
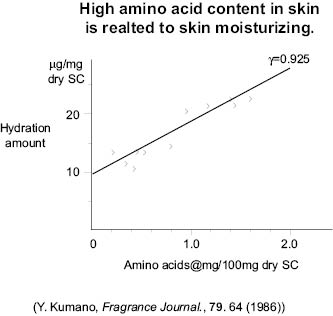
Figure 12
Since fully hydrated skin is soft and supple, the increase in hydration due to the presence of amino acids makes the skin softer and more pliable as is youthful skin.
The skin’s stratum corneum contains within the corneocytes the natural moisturizing factor (NMF) that helps the skin retain moisture and suppleness. This NMF is comprised of 40% amino acids, pyrrolidone carboxylic acid 12%, lactates 12%, urea 7%, ammonia, uric acid, glucosamine, creatinine, citrates, various salts, glucose, organic acids, peptides, and others totaling about 28% according to Spier and Pascher.19
The composition of the amino acids in the NMF is illustrated in the following graph.
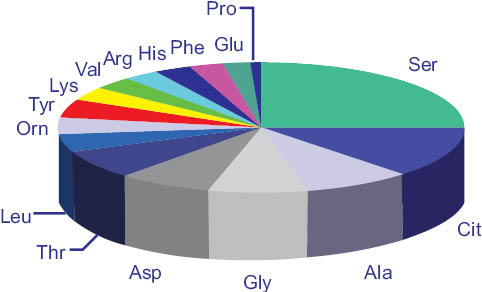
Figure 13
As mentioned above, there is a direct correlation between the amounts of amino acids present in the SC and the degree of skin hydration. The more amino acids present, the greater the hydration and the greater the suppleness of the skin.
Derivatives of amino acids can facilitate hydration or the mitigation of damage to the NMF. It has been shown that washing with a simple cleanser containing 10% active ingredient sodium lauryl ether sulfate will remove about 62% of the amino acids in the SC and ~56% of PCA present in the SC relative to the initial amounts present. Soap will remove about 70% amino acids and 62% PCA again relative to the initial amounts present. A wash with 10% active substance of sodium lauroyl glutamate, a glutamic acid derivative, washes out only 52% amino acids and 39% PCA of those initially present. This shows that the retention of amino acids and PCA can be influenced by the character of the surfactant employed to cleanse the skin. Similarly, the application of an amino acid–derived emollient can reduce the trans-epidermal water loss, thereby increasing hydration of the skin as reported by Ishii.20
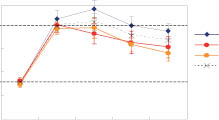
Figure 14
Where cholesteryl/behenyl/octyldodecyl lauroyl glutamate (golden line), and Type 3 ceramide (red line), petrolatum (black line), and untreated (blue line). The phytosterol analogs of the glutamic acid–based compounds show similar results. The timeframe is Initial, the Zero hour post-damage, then one, two, and three days after application of the treatment.
L-proline is unique among the amino acids in its ability the increase moisturization, especially in the presence of leucine and isoleucine.
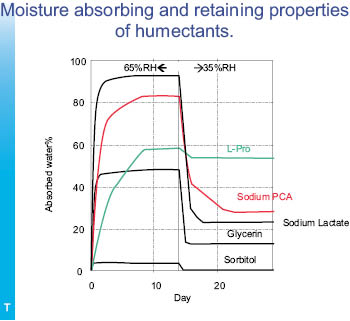
The graph above, Figure 15, contrasts the absorption of humectants at high-humidity and low-humidity conditions over the course of time. The humectants were initially dried to constant weight and then exposed to high, 65% RH over 15 days with the water weight uptake measured. At the end of day 15 the humectants were exposed to low, 35% RH for an additional 15 days with the water weight loss measured. The first case measures the greatest hydration from atmospheric moisture that can be expected to be attained while the second case measures the moisture retention under dry conditions.21
It can be easily seen that sodium PCA and sodium lactate absorb the greatest amount of moisture under high-humidity conditions but that L-proline retains the greatest amount of moisture relative to its initial hydration at low humidity. The low-humidity retention of moisture is important, since this is the condition that dries out the skin and causes it to be itchy and cracking.
Leucine and isoleucine establish a lipid bi-layer with water bound to L-proline. This bi-layer inhibits moisture loss from the skin.22
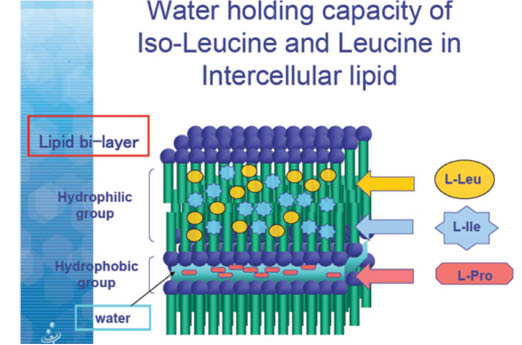
Figure 16
Amino acids and their derivatives are useful in mitigating the effects of UV radiation on the skin. It is important to note that these materials are not replacements for good sunscreens/sun blocks but augment their effects and protection.
Zinc PCA has been shown to prevent the formation of activator protein-1, which is caused by incident UV radiation. This inhibition prevents to a good extent the formation of collagenase and damage to collagen and elastin in the skin. Other amino acids such as cysteine, methionine, taurine, glutathione, and n-acetyl cysteine are powerful antioxidants and free-radical scavengers. When topically applied they augment the skin’s natural defenses against reactive oxygen species induced by UV radiation and other environmental factors.
Amino acid derivatives like the ester isopropyl lauroyl sarcosinate are outstanding solvents for organic UBV absorbers and allow easy incorporation of these materials into a formulation and help inorganic UV blocks spread uniformly across the skin substrate. Lauroyl lysine is another derivative that helps inorganic UV blocks deposit uniformly on the skin. It also has the advantage of delivering a soft lubricious skin feel.
Materials such as phytosterol/octyldodecyl lauroyl glutamate and phytosterol/behenyl/octyldoddecyl lauroyl glutamate are excellent wetting agents for pigments used as UV blocks, such as micronized titanium dioxide and zinc oxide. These also allow a more uniform deposition across the skin.
The function of amino acids in the hair is much less well understood than it is in the skin. Of course amino acids are constituents of keratin and an integral component of the cell membrane complex (CMC), which “glues” the cells of the cortex of the hair together.
We all start with healthy hair but with the modern propensity for washing, perming, and coloring, we damage it thereby causing breakage, dullness, loss of elasticity, and roughness. This damage shows up as lower tensile strength, cuticle damage, and components such as collagen, cholesterol, amino acids, fatty acids, and ceramides leaching out of the hair shaft. What are needed are materials that help repair the hair and/or mitigate the degree of damage in the first place. Amino acid derivatives can have a positive effect on the hair.
The reduction in tensile strength from perming and bleaching results in severe hair breakage. It has been shown that the tensile strength of permed hair is only 95% that of normal healthy hair and the tensile strength of bleached hair is only 90% of that of normal healthy hair. This reduction in tensile strength may seem inconsequential, but damage and breakage indeed occur.
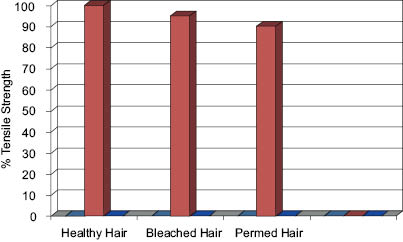
Fig. 17
In various tests it has been found that the addition of amino acid derivatives such as phytosterol/octyldodeyl lauroyl glutamate mitigates the damage done to hair from bleaching followed by shampooing. The testing procedure employed was to first take tensile strength readings on untreated hair in order to establish a baseline. Thereafter, hair was bleached with ammoniated peroxide in one case, bleached and shampooed with a 10% active sodium lauryl ether sulfate shampoo was employed in the second case, and in the third case the hair was bleached, and shampooed with a shampoo containing 0.5% phytosterol/octyldodecyl lauroyl glutamate. The untreated hair was normalized to 100% for comparison. The findings of these tests showed that bleached hair lost 5% of its tensile strength in the bleaching process, and that bleached and shampooed hair lost approximately 13% of its tensile strength. However, when 0.5 % of the amino acid derivative phytosteroyl/octyldodecyl lauroyl glutamate was added to the shampoo, the tensile strength of the hair returned to that of bleached only hair (95% of normal).
Similar effects were noted in permed hair that was first permed using an ammonium thioglycolate solution and then neutralized with a sodium bromide solution. Tensile strength measurements were made on permed hair in one case, on permed and sodium laureth sulfate–shampooed hair in a second case and on permed hair shampooed with phytosterol/octyldodecyl lauroyl glutamate added to the shampoo at 0.5% active. The results showed that permed hair retained only 75% of the tensile strength of normal hair and that shampooing further increased the damage so that the hair retained only 65% of its tensile strength. By contrast, the addition of the amino acid derivative prevented further damage from shampooing, allowing the hair to retain about 78% of its normal tensile strength.23
In additional tests hair was bleached with ammoniated peroxide, then conditioned with a commercial conditioner. The hair that was bleached and not conditioned had 85% of the tensile strength of normal unbleached hair, while the hair that was beached and conditioned with a commercial conditioner had 88% of the tensile strength of normal hair. The addition of 10% phytosterol/octyldodecyl lauroyl glutamate to the commercial conditioner restored the hair to 93% of its original normal tensile strength. The addition of phytosterol/octyldodecyl lauroyl glutamate and amino acid derivative to a shampoo or conditioner restores chemically damaged hair nearly to its original pre-damaged state.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree