26 Alveolar clefts
Synopsis
 Treatment of the alveolar cleft remains one of the most controversial topics in cleft care.
Treatment of the alveolar cleft remains one of the most controversial topics in cleft care.
 Treatment protocols have varied in timing, technique, and selection of graft material.
Treatment protocols have varied in timing, technique, and selection of graft material.
 Currently the gold standard is secondary bone grafting with autogenous cancellous graft at the time of mixed dentition.
Currently the gold standard is secondary bone grafting with autogenous cancellous graft at the time of mixed dentition.
 Outcomes of proposed alternate treatments such as gingivoperiosteoplasty (GPP), primary bone grafting, and use of inductive proteins need to be compared to documented outcomes of this gold standard.
Outcomes of proposed alternate treatments such as gingivoperiosteoplasty (GPP), primary bone grafting, and use of inductive proteins need to be compared to documented outcomes of this gold standard.
 Regardless of which technique is employed, coordination and communication between surgeon and orthodontist are essential for success.
Regardless of which technique is employed, coordination and communication between surgeon and orthodontist are essential for success.
 Failed or complex alveolar bone graft sites remain a considerable challenge. These recalcitrant alveolar clefts can benefit from recent applications of distraction osteogenesis techniques.
Failed or complex alveolar bone graft sites remain a considerable challenge. These recalcitrant alveolar clefts can benefit from recent applications of distraction osteogenesis techniques.
Introduction
• Compared to soft-tissue repair techniques, surgery on the bony alveolar defect in cleft lip and palate patients is relatively new.
• Early approaches including primary grafting in infancy fell into disfavor due to iatrogenic impairment of facial growth.
• Secondary bone grafting in mixed-dentition and primary GPP were introduced in the 1960s.
• Secondary bone grafting has become the gold standard for comparison of other techniques.
Historical perspective
Although the visible soft-tissue deformity associated with cleft lip and palate has been described and treated for centuries, the importance of treating the underlying skeletal pathology has only recently been realized. Primary bone grafting in infancy and early childhood was advocated by Schmid and others in the mid 20th century,1–5 but soon fell into disfavor, with long-term follow-up demonstrating iatrogenic impairment of facial growth. In the midst of this backlash to alveolar grafting, Boyne introduced the concept of secondary alveolar bone grafting in the 1960s, advocating treatment towards the end of the first decade of life to minimize growth impairment while still supporting eruption of the adult dentition.6 The same decade, Skoog published his experience with “boneless bone grafting,” which was the birth of the concept of GPP.7
All of these three treatment options of alveolar reconstruction – primary grafting, secondary grafting, and GPP – are currently practiced in modern cleft care; however most practitioners would accept that secondary bone grafting remains the most widely accepted or “gold standard.” Despite this predominance of secondary grafting, treatment of the alveolar cleft remains the most controversial topic of discussion at national cleft meetings.8 New technology in this century has introduced even more variables in the form of inductive proteins that could avoid the need for graft material, and devices that can generate bone formation through the principles of distraction osteogenesis.
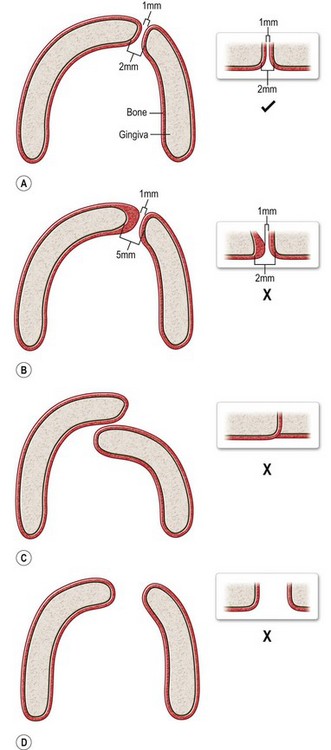
Practitioners in cleft care should understand the pros and cons of all options of alveolar cleft treatment regardless of their protocol of choice. Only in this way can the merits of new technologies and treatment protocols be fairly evaluated. The overriding goals of alveolar cleft treatment should be shared by all treatments and are listed in Table 26.1. The purpose of this chapter is to introduce the reader to the concepts of each treatment and to discuss the available literature regarding the success of each in achieving the treatment goals.
Table 26.1 Goals of treatment of the alveolar cleft
Basic science/disease process
Anatomy of the alveolar cleft
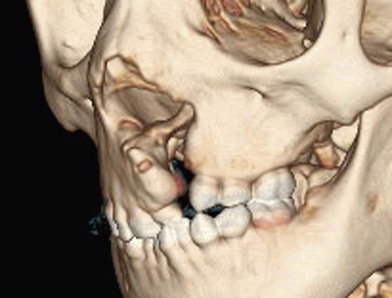
The alveolar cleft is more than a linear gap in the maxillary arch. With soft tissue removed the cleft is best visualized as a tornado, increasing in size from incisal to apical, becoming widest as it extends into the nasal cavity and distorts the surrounding anatomy (Fig. 26.1). The soft-tissue distortion caused by this skeletal deficiency can be minimized by a correctly performed cleft lip repair, but not completely eradicated. In the patient with an untreated alveolar cleft, the alar base of the nose lacks the bony support of the noncleft side, and if release of the lateral nasal wall and reconstruction of the nasal component of the orbicularis oris muscle have not been achieved at the primary lip repair, the nasal base will remain attached to the hypoplastic piriform rim, with inferior and posterior malposition.
Dental development
An alveolar cleft is associated with variable anomalies in dental development that must be taken into consideration with presurgical preparation, timing of surgery, surgical technique, and postsurgical orthodontic planning. Anomalies can include the number of teeth (missing teeth, supernumerary teeth), the location (mesial or distal to cleft), the shape (pegged or conical), the size (microdontic), the time of formation and/or eruption, and crown and root malformations.9,10
A goal of successful alveolar cleft treatment is to provide a stable supporting environment for eruption of the permanent canine. The adjacent lateral incisor however must be taken into consideration prior to surgery with a coordinated surgical–orthodontic plan. The lateral incisor is often missing in complete clefts; however, if present, it can be positioned either mesial or distal to the cleft. Although still debated, many consider that it is supernumerary when distal.11
It is important that the patient and family be informed before the bone graft procedure that in many cases a permanent lateral incisor is congenitally absent, will not erupt, or will be extracted as part of the treatment protocol.12–15 The lateral incisor is extracted if it is needed to create space for the permanent canine to migrate and erupt through the newly grafted area. The orthodontist can then perform canine substitution of the lateral incisor if it was absent or not supernumerary.
Diagnosis/patient presentation
Gingivoperiosteoplasty
Prior to undergoing a GPP, the infant with a cleft must be evaluated by the practitioner coordinating the presurgical molding as well as the surgeon who will be performing the GPP. It must be emphasized that not all infants will be candidates for GPP due to individual variations of anatomy. Some infants with particularly wide unilateral clefts can be “mesenchymally deficient.” Compressing and closing the alveolar cleft with molding and a GPP would unnaturally constrict the arch form. Isolated clefts of the primary palate are also difficult to predict if a GPP is possible. Due to the bony fusion of the secondary palate, the alveolar segments are more resistant to presurgical molding, and in some cases cannot be adequately aligned. Finally, in bilateral complete clefts, it is not always possible to align both sides of the premaxilla with the alveolar segments. In this case, one alveolar cleft can undergo a GPP to convert the arch form to a lesser and greater segment similar to a unilateral cleft. Assessment of parallel alveolar molding can be difficult, and benefits from a team presurgical evaluation (Fig. 26.2.) If the alveolar anatomy and presurgical molding outcome are favorable, a GPP can be offered to the family at the same time as the primary lip repair.
Primary bone grafting
Grafting at the time of primary dentition is practiced by relatively few centers, with the most published group being that of Rosenstein and Dado, who have used primary grafting as their approach to the alveolar cleft for over 20 years. Patient selection is based on the family’s ability to undertake the staged surgical and orthodontic appliance protocol necessary to maintain arch relationship before and after grafting.16
Secondary bone grafting
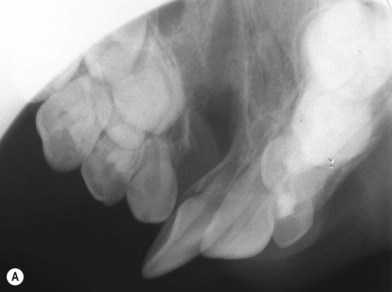
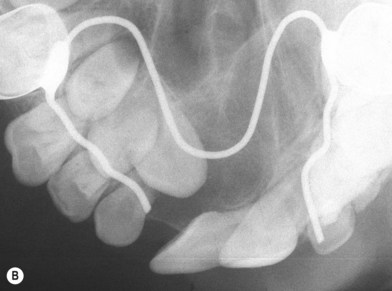
Debate continues regarding the appropriate timing of a secondary bone grafting. The mixed-dentition phase is variable among patients, but typically falls between ages 6 and 11. Proponents of grafting early in mixed dentition believe that a stable healed graft prior to canine eruption results in a superior bone environment (Fig. 26.3). El Deeb et al.17 found that successful eruption of cuspids though the graft occurred when root formation of the canine adjacent to the cleft was one-fourth to one-half formed at the time of graft placement. Bergland et al.18 found a higher proportion of graft failures and cases with lower interdental septa when grafting was done adjacent to fully erupted teeth compared to just before eruption. In comparison, Long et al. retrospectively performed detailed periapical radiograph analysis of bone formation and found no significant correlation between final graft success and the amount of canine crown eruption in the cleft at the time of grafting.19
Bone morphogenic protein
Recombinant human bone morphogenic protein-2 (rhBMP2) is a mitogen that has been demonstrated to stimulate osteoblastic activity and induce bone nodule formation in animals. It has been approved by the US Food and Drug Administration for clinical use in human spine fusion procedures, and has been shown to decrease nonunion, donor site morbidity, and operating time over autogenous grafting in this population.20 More recent clinical applications have been on patients undergoing alveolar augmentation and implant placement21 and early trials are now underway at individual centers for the treatment of alveolar clefts. The risk–benefit profile of rhBMP2 in these patients will remain unknown for the next decade. Patient selection should therefore be based on enrolment in an institutional review board-approved trial with appropriate consent and evaluation, including oversight by an independent data safety monitoring board.
Late bone grafting
Some centers have advocated delaying alveolar bone grafting in patients who are known to need a LeFort I osteotomy procedure at skeletal maturity in order to perform both simultaneously. This however has largely been abandoned in the presence of an erupting canine, since eruption into a bone graft is felt to provide better bone support and periodontal status.12
Alveolar distraction
Successful primary or secondary treatment of the alveolar cleft should obviate the need for alveolar distraction in this patient population. Unfortunately there exist patients with “ungraftable” or “recalcitrant” alveolar clefts that have few options available to them other than undergoing transport distraction osteogenesis (TDO) of alveolar bone.22 The typical patient who falls into this category has unhealthy, scarred gingiva, a large nasolabial and/or oronasal fistula, and a history of repeated unsuccessful bone grafts with infections and exposure. Another possible presentation is a previously grafted maxilla that has severe vertical deficiency along with scarred mucogingiva preventing additional graft augmentation. In both these cases, TDO is a useful tool to have in the cleft armamentarium.
In the wide “ungraftable” cleft patient (Fig. 26.4), a tooth-bearing transport segment can be slowly moved into the gap, closing the fistula and converting the problem into a narrow cleft amenable to traditional secondary grafting. In the vertically deficient alveolus (Fig. 26.5), a transport segment of superior maxillary bone, with or without prior bone augmentation, can be slowly lowered, leveling the alveolar ridge by gradually bringing the scarred attached gingival tissue along with the advancing bone front.
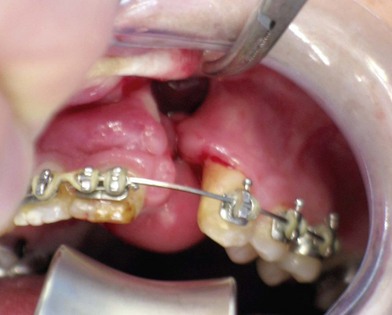
Fig. 26.4 A good candidate for interdental horizontal transport distraction. The patient had undergone three previous attempts at treatment of bilateral alveolar clefts, including transfer of a facial artery musculomucosal (FAMM) flap, seen hanging from the anterior palate. The patient has extensive scarring and a large tornado-shaped oronasal and nasolabial fistula. The recommended treatment was simultaneous closure of the nasal lining using the FAMM flap tissue with transport distraction of a three-tooth segment from the lesser segments, as shown in Figure 26.9, followed by secondary bone grafting of the residual cleft.
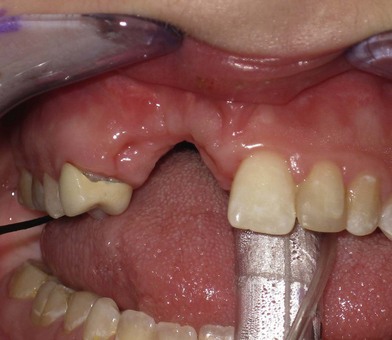
Fig. 26.5 A good candidate for vertical transport distraction. The patient had undergone six previous attempts of alveolar grafting with infection and graft loss. The result was vertical deficiency of the alveolus, loss of the adjacent three permanent teeth, and tight scarred gingival covering. The patient underwent stage cortical grafting and vertical transport distraction, as shown in Figure 26.11.