Albinism and Other Genetic Disorders of Pigmentation: Introduction
|
Epidemiology
Oculocutaneous albinism (OCA) is the most common inherited disorder of generalized hypopigmentation, with an estimated frequency of 1 in 20,000 in most populations. Four different types of OCA have been described. OCA1 and OCA2 are the most frequent types and account for approximately 40% and 50%, respectively, of OCA worldwide. OCA2 occurs in approximately 1 in 30,000 to 1 in 36,000 Caucasians and 1 in 10,000 to 1 in 17,000 blacks in the United States,1–3 but is reported at higher frequencies ranging from 1 in 1,400 to 1 in 7,000 in Sub-Saharan Africa4,5 and even as high as 1 in 170 individuals in the Kuna population of the Panama coast.6 OCA3 and OCA4 are far less frequent, although the rufous OCA phenotype, described later, associated with OCA3 in South African blacks has been reported at an incidence of approximately 1/8,500,7 while OCA4 accounts for 27% of all cases of OCA in Japan.8
Hermansky–Pudlak syndrome (HPS) is rare except in the Caribbean island of Puerto Rico, particularly in the northwestern region where the majority of patients are found, with an incidence of 1 in 1,800.9 Chediak–Higashi syndrome is also quite rare.
Waardenburg syndrome (WS) is probably less frequent than OCA. The highest reported incidence is 1/20,000 in Kenya, although most estimates of its incidence in the Netherlands where it was originally reported are in the range of 1/40,000. The incidence of WS with deafness is lower, ranging between 1/50,000 and 1/212,000. WS has been described occurring in a range of frequencies in the congenitally deaf, ranging from 0.9%–2.8% to 2%–5% in different reports. The incidence of piebaldism is estimated to be less than 1/20,000.10,11
Etiology, Pathogenesis, and Clinical Features
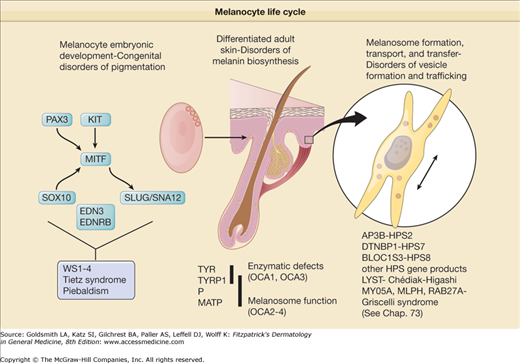
Awareness of the biologic basis of the distinction between congenital disorders of pigmentation, which are disorders of melanocyte development, and the varieties of albinism, which are disorders of melanocyte differentiation, is important for fully understanding their clinical manifestations. Albinism results from the dysfunction of a normal complement of pigment cells, which results in complete or partial loss of cutaneous pigmentation. The forms of albinism, including the subtypes of OCA as well as albinism syndromes with systemic manifestations, result either from enzymatic defects in the biosynthesis of melanin, from melanosomal defects that interfere with melanin formation, or from problems in the intracellular transport and localization of proteins essential for melanin biosynthesis (Table 73-1; see Chapter 72). Congenital disorders of pigmentation usually result from mutations in genes critical for melanocyte development during embryogenesis. These disorders can also be associated with other systemic problems because of the requirement for these gene products in the development of cell types other than melanocytes. More accurate descriptors of these disorders could be congenital (or genetic) disorders of melanocyte differentiation and congenital (or genetic) disorders of melanocyte development, to reflecting the fact that both categories of conditions, albinism and developmental pigmentary syndromes, are generally inherited but result from different mechanisms of disease (Fig. 73-1).
Disease | Chromosome | Gene Locus | Protein [Biogenesis of Lysosome-Related Organelles Complex (BLOC)] | Distinguishing Signs |
|---|---|---|---|---|
Oculocutaneous albinism type 1 | 11q21 | TYR | Tyrosinase/TYR | Type 1A—no pigment Type 1B—some pigment |
Oculocutaneous albinism type 2 | 15q11.2-q12 | P | Pink protein/P | Some pigmentation apparent; nevi and freckles possible |
Oculocutaneous albinism type 3 | 9p23 | TYRP1 | Tyrosinase-related protein-1/TYRP1 | Similar to OCA2 phenotype. Includes rufous (red) albinism phenotype. |
Oculocutaneous albinism type 4 | 5p | MATP | Membrane-associated transporter protein/MATP | Similar to OCA2 phenotype. Most common in individuals with Asian biogeographic ancestry. |
Hermansky–Pudlak syndromes | ||||
Type 1 | 10q24 | HPS1 | HPS1 (BLOC-3) | Pulmonary fibrosis associated; absent platelet dense granules is a hallmark of all types of HPS |
Type 2 | 15q15 | AP3B1 | β-3 subunit of adaptor protein 3 complex/AP3B1 | |
Type 3 | 3q24 | HPS3 | HPS3 (BLOC-2) | |
Type 4 | 22q11.2-q12.2 | HPS4 | HPS4; distant homology with yeast CCZ1 (BLOC-3) | Pulmonary fibrosis associated |
Type 5 | 11p14 | HPS5 | HPS5 (BLOC-2) | |
Type 6 | 10q24.32 | HPS6 | HPS6 (BLOC-2) | |
Type 7 | 6p22.3 | DTNBP1 | Dysbindin (BLOC-1) | |
Type 8 | ? | BLOC1S3 | BLOC1S3 (BLOC-1) | |
Chediak–Higashi syndrome | 1q43 | LYST | Lysosome trafficking/LYST | Giant peroxidase-positive lysosomal granules in neutrophils |
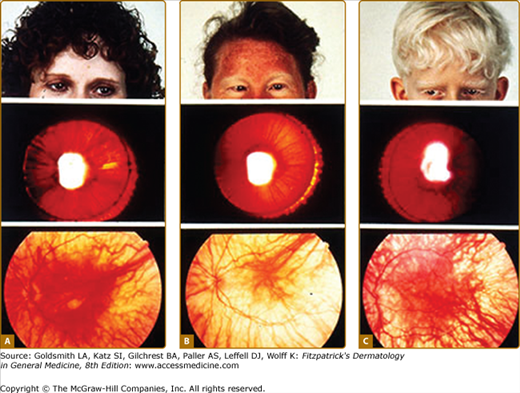
Although the pigmentary abnormalities associated with types of albinism can vary widely, common to all types of albinism is reduced visual acuity and ocular nystagmus, a result of misrouting of the optic nerve at the optic chiasm and foveal hypoplasia. This has been described not only in humans12 but also in other albino mammals.13 Experiments using the tyrosinase promoter to express both tyrosinase and tyrosine hydroxylase in albino transgenic mice suggest that the tyrosine hydroxylase activity of tyrosinase is particularly important for ensuring the proper routing of retinal projections at the optic chiasm during development.14,15 The ocular manifestations of albinism can vary greatly, ranging from severe (20/400) to nearly undetectable, but is often close to 20/80.16 They also include a reduction in iris pigment, a reduction in retinal pigment, and alternating strabismus.
OCA1 [Online Mendelian Inheritance in Man (OMIM) #203100] is caused by loss of function of the melanocytic enzyme tyrosinase resulting from mutations of the TYR gene.17–19 Null mutations are associated with a total loss of function and no pigment formation (OCA1A), whereas “leaky” mutations result in an enzyme that retains some function and is associated with some pigment formation (OCA1B). OCA1 appears to be the most common type of albinism in non-Hispanic Caucasian patients.20
Analyses of DNA from individuals with OCA1A have shown a large number of different mutations in the TYR gene. These mutations include missense, nonsense, frameshift, splice site, and deletion mutations. Most individuals with OCA1 are compound heterozygotes with different mutant maternal and paternal alleles.7 Missense mutations in the TYR gene are distributed among distinct regions of the coding sequence, which suggests that the encoded protein has multiple functional domains. Two of the clusters are in the copper-binding regions, with a third near the amino-terminus of the mature protein, in the extramelanosomal domain of tyrosinase shown to require phosphorylation for enzyme activation.21,22 Clustering of mutations in discrete regions of the coding sequence is consistent with these regions’ importance either for the melanogenic activity of tyrosinase or for functions related to its maturation or processing.7,22 Missense mutations at the signal peptide cleavage site23,24 implicate this cleavage event as an important step in the development of tyrosinase activity. Frameshift mutations near the C-terminus of the coding region25,26 indicate that the cytoplasmic domain of tyrosinase is also important for full activity, possibly because of the presence of protein kinase C-β-dependent phosphorylation sites that have been identified at its extreme C-terminus.22
All nonsense and frameshift mutations are associated with a complete loss of tyrosinase activity, presumably because of the subsequent production of a truncated protein. With missense mutations the picture is more complicated. A set of missense mutations that were associated with OCA1 patients accumulating pigment with age in either OCA1B or temperature-sensitive OCA (OCA1TS) patients27 were shown to have residual enzymatic activity. Hence, it is likely that a subset of TYR missense mutations are responsible for the OCA1B and OCA1TS phenotypes because of the reduced, rather than absent, tyrosinase activity in their melanocytes.7 However, other missense mutations resulting in the OCA1A and OCA1TS phenotypes result in defective intracellular processing of tyrosinase and retention of the mutant tyrosinase proteins in the endoplasmic reticulum, which suggests that some molecular variants of OCA1 represent an endoplasmic reticulum retention disease.28–30 Thus, the residual enzymatic activity of a missense tyrosinase mutant cannot fully predict its phenotype, because other, presumably conformational, determinants of nascent mutant proteins may lead to their retention in the endoplasmic reticulum, block transport to the melanosome, and cause a more severe pigmentary phenotype.
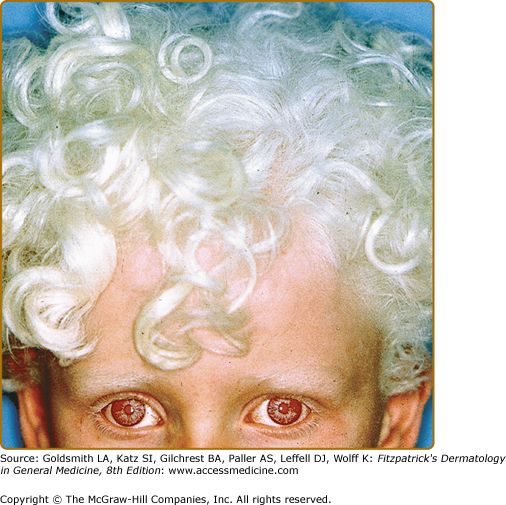
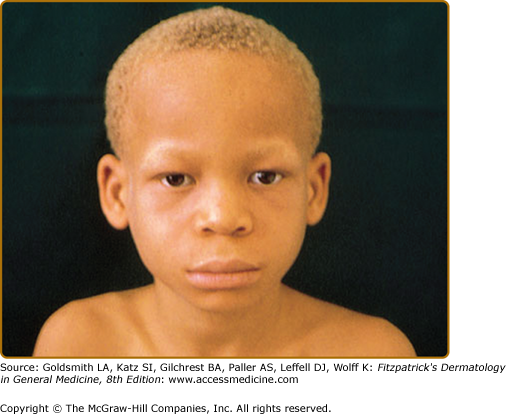
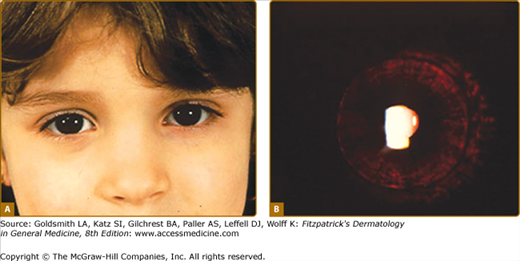
In OCA1A, or the classic tyrosinase-negative OCA, there is a complete inability to synthesize melanin in skin, hair, and eyes, resulting in the characteristic “albino” phenotype. Affected individuals are born with white hair and skin and blue eyes, and there are no changes as they mature. The phenotype is the same in all ethnic groups and at all ages (Fig. 73-2). The hair may develop a slight yellow tint due to denaturing of the hair protein due to sun exposure and/or shampoo use. The irides are translucent, appear pink early in life, and often turn a gray–blue color with time. No pigmented lesions develop in the skin, although amelanotic nevi can be present. The architecture of skin and hair bulb melanocytes is normal. The melanosomes show a normal melanosomal membrane, and normal internal matrix formation is observed in stage 1 and 2 melanosomes.
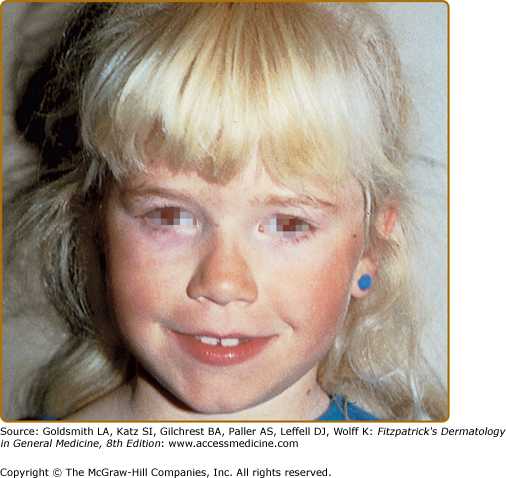
The phenotype of OCA1B can range from minimal hair pigment to skin and hair pigmentation approaching the normal pigmentary phenotype for the individual’s genetic composition and continental ancestry. Most individuals with OCA1B have very little or no pigment at birth and develop varying amounts of melanin in the hair and skin in the first or second decade of life (Fig. 73-3). In some cases the melanin develops within the first year. The hair color changes to light yellow, light blond, or golden blond first, as a result of residual pheomelanin synthesis, and eventually can turn dark blond or brown in adolescents and adults. The irides can develop light-tan or brown pigment, sometimes limited to the inner third of the iris, and iris pigment can be present on globe transillumination. However, some degree of iris translucency, as demonstrated by slit lamp examination, is usually present. Many individuals with OCA1B will tan with sun exposure, although it is more common to burn without tanning. Pigmented lesions (nevi, freckles, lentigines) develop in the skin of individuals who have developed pigmented hair and skin. In some patients, the moderate amount of residual tyrosinase activity can lead to near-normal cutaneous pigmentation, so that the clinician may overlook subtle cutaneous pigmentary abnormalities and render instead the mistaken diagnosis of ocular albinism (OA).
One variation of OCA1B is the temperature-sensitive phenotype. In this variation, scalp and axillary hair remain white or slightly yellow, but arm and leg hair pigments. The skin remains white and does not tan. The retention of melanin synthesis in the cooler areas of the body, such as the arms and legs, but not the warmer areas, such as the trunk and the scalp, is associated with a temperature-sensitive mutation in tyrosinase, which loses activity above 35°C.27 Similar tyrosinase mutations have been described in the Himalayan mouse31 and in the Siamese cat with dark “points” at the tips of the ears and on the paws.32
Mutations of the OCA2 (P) gene, which maps to chromosome arm 15q, are responsible for OCA2 (OMIM #203200).33 OCA2 occurs worldwide, though somewhat more frequently in the African, African-American, and certain Native American populations. Historically, affected individuals have benefited from limiting their sun exposure, especially in desert and equatorial climates. Interesting anthropological studies have described how various societies have differed in their treatment of members with OCA2. For example, the Cuna Indians of Panama actively forbade marriage between female and male albinos, and infanticide against albino infants was common in the early twentieth century. In contrast, no marriage discrimination was practiced in Hopi tribes, whose albinos were not expected to participate in farming activities requiring substantial exposure to sunlight.34 From the standpoint of melanin synthesis, the defect in OCA2 appears to involve a reduction in eumelanin synthesis primarily, with less effect on pheomelanin synthesis. The predicted structure of the OCA2 gene, a melanosomal protein, includes 12 transmembrane domains.35,36 As expected, a number of mutations of the human OCA2 gene are associated with human OCA2.37,38
In Sub-Saharan Africa, a single 2.7-kb deletion allele accounts for 60%–90% of mutant OCA2 alleles and is associated with a common haplotype, suggesting a common founder.5,38–40 It has been estimated that this single mutation is associated with 25%–50% of all mutant OCA2 alleles in African-Americans.5,38–44 However, other diverse mutant alleles have been described in this population and in Africans. The Brandywine, Maryland, isolate affects an inbred American population, originally located in a rural area east of Washington, DC, that has been studied extensively for its prevalence of albinism, dentinogenesis imperfecta, and other inherited recessive and dominant conditions, with mixed Caucasian, African, and possibly Native American ancestry.45 In this population, 1 in 85 individuals has OCA245,46 and is homozygous for the 2.7-kb deletion allele of OCA2.38 Thus, it is likely that this 2.7-kb deletion allele accounts for the distinct OCA2 phenotype in Africans and African-Americans.47
OCA2 also has been reported at relatively high frequencies ranging from 1 in 28 to 1 in 6,500 in specific Native American groups, including those populations in the southwest United States (Hopi population), southern Mexico, eastern Panama (Kuna population), and southwest Brazil.34 In the Navajo population, a homozygous 122.5-kb deletion has been described in members with OCA2. This mutations results in the loss of exons 10 to 20 of OCA2, corresponding to a region containing seven of the transmembrane domains, and appears to be specific for OCA2 within the Navajo population.48 On the other hand, OCA2 in the Kuna population is caused by a splice site mutation in intron 17 of OCA2.49 Unlike the mutations in TYR, the missense mutations described to date in OCA2 do not seem to cluster in any specific region.
Regarding OCA2 gene product function, it has been shown that melanosomes from P protein-deficient melanocytes have an abnormal pH. Melanosomes in cultured melanocytes derived from wild-type mice are typically acidic, whereas melanosomes from P protein-deficient mice are nonacidic.50 Hence, it is likely that the P protein regulates the pH of melanosomes, perhaps by functioning as an anion cotransporter in conjunction with a distinct proton pump on the melanosomal membrane. An alternate possibility is that the acidic conditions mediated by the P protein favor the normal biogenesis of melanosomes, including the correct targeting of other melanosomal proteins such as tyrosinase.51 Single nucleotide polymorphisms in the first intron of OCA2 are the major determinant of brown versus blue iris color,52 and a germ line polymorphism of the OCA2 gene is associated with favorable survival of estrogen receptor-negative breast cancer.53 Conceivably this finding may be related to the previously reported enhanced sensitivity of cells overexpressing P protein to cytotoxic agents.54
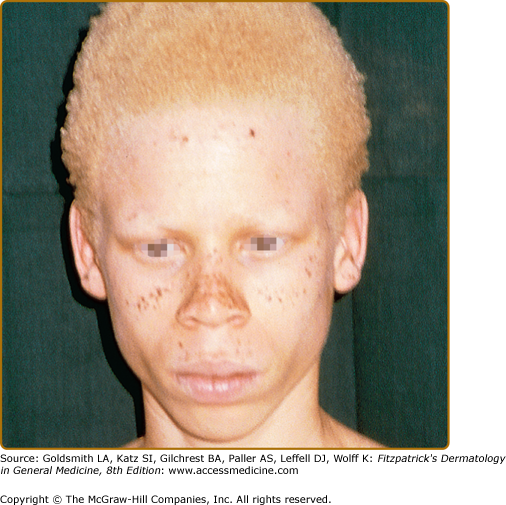
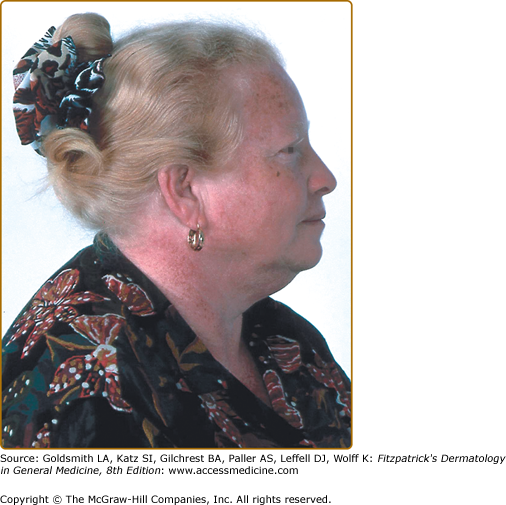
In African and African-American individuals, there is a distinct OCA2 phenotype (Fig. 73-4). Hair is yellow at birth and remains so throughout life, although the color may turn darker. Hair color can turn lighter in older individuals, and this probably represents the normal graying with age. The skin is creamy white at birth and changes little with time. No generalized skin pigment is present, and no tan develops with sun exposure, but pigmented nevi, lentigines, and freckles often develop, since the cutaneous melanocytes in these individuals both remain susceptible to ultraviolet (UV)-induced changes early in life and retain some ability to synthesize melanin later. The irides are blue–gray or light tan or brown. The development of lentigines or ephelides, well-demarcated pigmented patches usually on sun-exposed areas of the skin, may be evidence of a separate genetic susceptibility because these lesions only develop in some OCA2 families and not in others. The presence versus absence of ephelides is associated with a lower risk of skin cancer in South African albinos,55 probably because the ability to produce ephelides by albinos in that environment also signifies a greater ability to produce pigment and thus demonstrate increased protection from UV radiation.
![]() The brown OCA phenotype is a distinct OCA2 phenotype, which has been described in the African and African-American populations. In this clinically less severe phenotype,56 the hair and skin color are light brown and the irides are gray-to-tan at birth (eFigure 73-6.1). With time, there is little change in skin color, but the hair may turn darker, and the irides may accumulate more tan pigment. The skin generally does not burn but may darken with sun exposure. Affected individuals are recognized as having albinism rather than a variation in normal pigmentation because of the ocular changes present. The iris has punctate and radial translucency, and moderate retinal pigment is present. Visual acuity ranges from 20/60 to 20/150. In brown OCA, the amount of eumelanin in the skin and hair is reduced but not absent. Recent studies have shown that brown OCA is associated with heterozygosity for P gene alleles, one of which is null and the other having partial function.56
The brown OCA phenotype is a distinct OCA2 phenotype, which has been described in the African and African-American populations. In this clinically less severe phenotype,56 the hair and skin color are light brown and the irides are gray-to-tan at birth (eFigure 73-6.1). With time, there is little change in skin color, but the hair may turn darker, and the irides may accumulate more tan pigment. The skin generally does not burn but may darken with sun exposure. Affected individuals are recognized as having albinism rather than a variation in normal pigmentation because of the ocular changes present. The iris has punctate and radial translucency, and moderate retinal pigment is present. Visual acuity ranges from 20/60 to 20/150. In brown OCA, the amount of eumelanin in the skin and hair is reduced but not absent. Recent studies have shown that brown OCA is associated with heterozygosity for P gene alleles, one of which is null and the other having partial function.56
eFigure 73-6.1
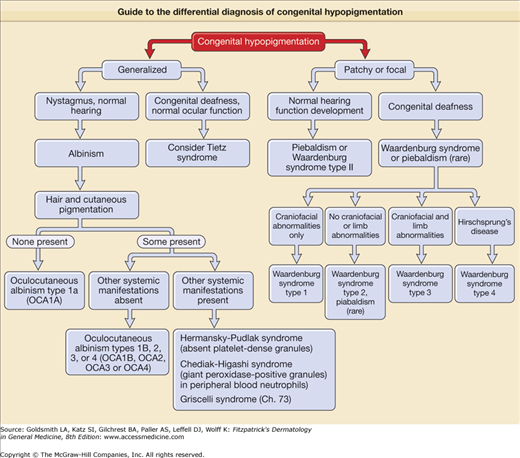
Guide to the differential diagnosis of congenital hypopigmentation. Generalized congenital hypopigmentation, with the exception of Tietz syndrome, is usually associated with oculocutaneous albinism (OCA), either one of the classic subtypes of OCA (OA 1–4), or one of the forms of OCA with systemic manifestations such as Hermansky–Pudlak syndrome. Patchy or focal congenital hypopigmentation is typically associated with a syndrome characterized by defective melanocyte development such as piebaldism or Waardenburg syndrome.
In Caucasian individuals with OCA2, the amount of hair pigment present at birth or developing with time varies from minimal in northern Europeans (particularly Scandinavians) to moderate in southern European or Mediterranean individuals. The hair can be very lightly pigmented at birth, having a light yellow or blond color, or more pigmented with a definite blond, golden blond, or even red color. The skin is creamy white and does not tan. The iris color is blue–gray or lightly pigmented, and the amount of translucency correlates with the development of iris pigment. With time, pigmented nevi and lentigines may develop, and freckles are seen in areas with repeated sun exposure. The hair in Caucasian individuals may slowly turn darker through the first two or more decades of life.
The normal delayed maturation of the pigment system and sparse hair early in life can make it difficult to recognize albinism early in northern European individuals. For all types of OCA in northern European families, the cutaneous hypopigmentation at birth or early in life is often similar to that of the parents and relatives, and the first concern is raised when it appears that the child is not tracking well or has developed nystagmus.
Prader–Willi and Angelman syndromes often are associated with hypopigmentation.57,58 The intragenic deletion encompassing one P allele in these patients59,60 suggests that the observed pigmentary phenotype is related to OCA2 and the P gene, even if the details of this association are not fully understood.47
Prader–Willi and Angelman Syndromes
![]() Prader–Willi and Angelman syndromes, whose clinical manifestations are described later but which can include cutaneous hypopigmentation, are associated with paternal or maternal, respectively, deletions of regions of the long arm of chromosome 15 containing a portion of the P gene. Hypopigmentation in these patients, who remain hemizygous for the P gene in the context of their large 15q deletion, suggests that there may be other genetic determinants of pigmentation in the chromosome 15 region of the P gene. Less commonly, these syndromes are associated with chromosome 15q uniparental disomy, or the exclusive presence of two copies of 15q from one parent, with maternal disomy associated with Prader–Willi’s syndrome and paternal disomy associated with Angelman’s syndrome.3 In addition, Prader–Willi’s syndrome or Angelman’s syndrome patients with OCA2 have been described with a typical deletion of one homolog of the P gene in the context of Prader–Willi’s syndrome or Angelman’s syndrome and inheritance of a mutation on the another homolog.61
Prader–Willi and Angelman syndromes, whose clinical manifestations are described later but which can include cutaneous hypopigmentation, are associated with paternal or maternal, respectively, deletions of regions of the long arm of chromosome 15 containing a portion of the P gene. Hypopigmentation in these patients, who remain hemizygous for the P gene in the context of their large 15q deletion, suggests that there may be other genetic determinants of pigmentation in the chromosome 15 region of the P gene. Less commonly, these syndromes are associated with chromosome 15q uniparental disomy, or the exclusive presence of two copies of 15q from one parent, with maternal disomy associated with Prader–Willi’s syndrome and paternal disomy associated with Angelman’s syndrome.3 In addition, Prader–Willi’s syndrome or Angelman’s syndrome patients with OCA2 have been described with a typical deletion of one homolog of the P gene in the context of Prader–Willi’s syndrome or Angelman’s syndrome and inheritance of a mutation on the another homolog.61
![]() Prader–Willi’s syndrome is a developmental syndrome that includes neonatal hypotonia, hyperphagia and obesity, hypogonadism, small hands and feet, and mental retardation associated with characteristic behavior. Many individuals with Prader–Willi’s syndrome are hypopigmented but do not have the typical ocular features of albinism; however, individuals with Prader–Willi’s syndrome can have typical features of OCA. For those without OCA, the hypopigmentation is characterized by hair and skin that are lighter than unaffected family members. Childhood nystagmus and strabismus are common but often do not persist into adult life. The irides are pigmented with some translucency on globe transillumination, and retinal pigment is reduced in amount. Foveal hypoplasia usually is not present, but the fovea may not appear entirely normal. Visual evoked potential studies have revealed optic tract misrouting similar to that found in albinism in some individuals with Prader–Willi’s syndrome and hypopigmentation, but this is not a universal finding. Some individuals with Prader–Willi’s syndrome have typical OCA2 with cutaneous hypopigmentation and all ocular features of albinism.
Prader–Willi’s syndrome is a developmental syndrome that includes neonatal hypotonia, hyperphagia and obesity, hypogonadism, small hands and feet, and mental retardation associated with characteristic behavior. Many individuals with Prader–Willi’s syndrome are hypopigmented but do not have the typical ocular features of albinism; however, individuals with Prader–Willi’s syndrome can have typical features of OCA. For those without OCA, the hypopigmentation is characterized by hair and skin that are lighter than unaffected family members. Childhood nystagmus and strabismus are common but often do not persist into adult life. The irides are pigmented with some translucency on globe transillumination, and retinal pigment is reduced in amount. Foveal hypoplasia usually is not present, but the fovea may not appear entirely normal. Visual evoked potential studies have revealed optic tract misrouting similar to that found in albinism in some individuals with Prader–Willi’s syndrome and hypopigmentation, but this is not a universal finding. Some individuals with Prader–Willi’s syndrome have typical OCA2 with cutaneous hypopigmentation and all ocular features of albinism.
![]() Angelman’s syndrome is a complex developmental disorder with developmental delay and severe mental retardation, microcephaly, neonatal hypotonia, ataxic movements, and inappropriate laughter. In Angelman’s syndrome, the hypopigmentation is characterized by light skin and hair. Nystagmus or strabismus may be present early in life, and iris translucency and reduced retinal pigment may be present. No analysis of optic nerve formation is available. As in Prader–Willi’s syndrome, individuals with Angelman’s syndrome who have typical OCA2 features have been described.62
Angelman’s syndrome is a complex developmental disorder with developmental delay and severe mental retardation, microcephaly, neonatal hypotonia, ataxic movements, and inappropriate laughter. In Angelman’s syndrome, the hypopigmentation is characterized by light skin and hair. Nystagmus or strabismus may be present early in life, and iris translucency and reduced retinal pigment may be present. No analysis of optic nerve formation is available. As in Prader–Willi’s syndrome, individuals with Angelman’s syndrome who have typical OCA2 features have been described.62
Mutations in the TYRP1 gene result in OCA3 (OMIM #203290). The first described mutation was in an African-American newborn twin initially classified clinically as brown OCA. Mutation analysis revealed a single-base deletion at codon 368 producing a frameshift and premature stop codon in exon 6 and a slightly truncated TYRP1 molecule.63 This mutation is shared by a substantial proportion of the rufous (“red”) OCA population in southern Africa.64 Rufous OCA is a distinct OCA phenotype in which the skin color is a mahogany brown with a slight reddish hue, and the hair color varies from deep mahogany to sandy red.2,64 Additional OCA3-associated TYRP1 mutations include a single-base substitution at codon 166, resulting in the alteration of a serine to a premature stop codon in exon 3 and a truncated TYRP1 molecule,64 also identified in the rufous OCA population; and, in a Pakistani kindred, individuals homozygous for a distinct premature termination mutation.65 A Caucasian male was compound heterozygous for a missense mutation in TYRP1 located in the second copper-binding domain, inherited from the patient’s mother, and a stop codon, which apparently occurred spontaneously.66 Interestingly, the p.S166X mutation in TYRP1 previously associated with rufous OCA64 was found to modify an OCA2 phenotype to a red-haired variant.67
OCA3 has presented with both the brown OCA and the rufous OCA phenotypes in the African and African-American populations. In the two cases of individuals with OCA3 mutations only, not of African descent, the phenotype has been that of a tyrosinase-positive albinism, such as OCA1B or OCA2. As additional examples of OCA3 are characterized, genotype–phenotype correlation should become clearer.
Pathogenesis of OCA3
![]() The pathogenesis of OCA3 remains poorly understood because the normal function of Tyrp1 in human melanocytes is still being clarified. In murine melanocytes, Tyrp1 protein appears to function as DHICA oxidase (Kobayashi et al, 1994). However, this enzymatic activity has not been observed for human Tyrp1 protein.68,69 Clearly Tyrp1 is important for melanosomal melanization, not only because of the phenotype of the brown mouse, with a TYRP1 mutation and accompanying coat color dilution, but also because of the pigmentary phenotypes of patients with OCA3 due to TYRP1 mutations. TYRP1 and tyrosinase reciprocally stabilize each other through associations in the endoplasmic reticulum, with mutations in either of them resulting in the retention and degradation of both in the endoplasmic reticulum, thus reducing their transport to melanosomes.70 This suggests that abnormal trafficking and/or reduced stability of wild-type tyrosinase elicited by mutant Tyrp1 could be an important etiologic factor of OCA3.
The pathogenesis of OCA3 remains poorly understood because the normal function of Tyrp1 in human melanocytes is still being clarified. In murine melanocytes, Tyrp1 protein appears to function as DHICA oxidase (Kobayashi et al, 1994). However, this enzymatic activity has not been observed for human Tyrp1 protein.68,69 Clearly Tyrp1 is important for melanosomal melanization, not only because of the phenotype of the brown mouse, with a TYRP1 mutation and accompanying coat color dilution, but also because of the pigmentary phenotypes of patients with OCA3 due to TYRP1 mutations. TYRP1 and tyrosinase reciprocally stabilize each other through associations in the endoplasmic reticulum, with mutations in either of them resulting in the retention and degradation of both in the endoplasmic reticulum, thus reducing their transport to melanosomes.70 This suggests that abnormal trafficking and/or reduced stability of wild-type tyrosinase elicited by mutant Tyrp1 could be an important etiologic factor of OCA3.
![]() OCA4 (OMIM #606574) is a form of oculocutaneous albinism (OCA) that may be rather rare worldwide, but relatively common as a type of albinism in East Asian populations. A variety of mutations, including a splice-acceptor-site mutation and missense mutations, have been found in a gene called MATP (membrane-associated transporter protein), located on chromosome 5p, in OCA4.71,73 OCA4 can have a variable phenotype, ranging from absence of pigmentation to some pigmentation with brown irides. Pigment darkening during the first decade of life has been reported.73
OCA4 (OMIM #606574) is a form of oculocutaneous albinism (OCA) that may be rather rare worldwide, but relatively common as a type of albinism in East Asian populations. A variety of mutations, including a splice-acceptor-site mutation and missense mutations, have been found in a gene called MATP (membrane-associated transporter protein), located on chromosome 5p, in OCA4.71,73 OCA4 can have a variable phenotype, ranging from absence of pigmentation to some pigmentation with brown irides. Pigment darkening during the first decade of life has been reported.73
![]() The protein product of the MATP gene is predicted to be a membrane protein spanning the membrane 12 times, and containing a conserved sucrose transporter signature sequence, suggesting an important functional role for this motif.72 In addition, melanosome anomalies have been observed in the medaka fish and in the mouse homologs of OCA4.72,74 These data indicate that MATP/Matp plays a critical role in vertebrate pigmentation and suggest that MATP/Matp may be a component of the melanosomal membrane, presumably mediating the transport of a molecule required for melanogenesis or for another melanosome function.
The protein product of the MATP gene is predicted to be a membrane protein spanning the membrane 12 times, and containing a conserved sucrose transporter signature sequence, suggesting an important functional role for this motif.72 In addition, melanosome anomalies have been observed in the medaka fish and in the mouse homologs of OCA4.72,74 These data indicate that MATP/Matp plays a critical role in vertebrate pigmentation and suggest that MATP/Matp may be a component of the melanosomal membrane, presumably mediating the transport of a molecule required for melanogenesis or for another melanosome function.
Mutations in eight different genes to date have been associated with types of HPS.70 Currently, our understanding about the function of their gene products varies greatly. However, a common theme is their functional involvement in trafficking cell type-specific products in cells containing lysosome-related organelles (LROs), including melanosomes in melanocytes.
HPS patients have OCA, with variable hypopigmentation of the skin, hair, and irides, and ocular abnormalities (see Fig. 73-5 and eFigs. 73-4.1, 73-5.1, 73-5.2, and 73-5.3). In addition, they lack platelet dense bodies and demonstrate a prolonged bleeding time, mucous membrane bleeding, a predisposition to epistaxis, easy bruising, and metromenorrhagia.75 Whole-mount electron microscopy is used to provide a definitive determination of the absence of platelet dense bodies.76
The greatest clinical experience exists with patients with HPS1 (OMIM #604982), HPS3 (OMIM #606118), and HPS4 (OMIM #606118). Pulmonary fibrosis is a common and severe manifestation of HPS1 and HPS4, generally causing death between the fourth and sixth decades of life.70,77 However, pulmonary fibrosis appears not to be associated with HPS3, which also features less severe pigmentary abnormalities.78–80 Among HPS1 and HPS4 patients, a granulomatous colitis is associated, occurring in approximately 15%.81,77 Ceroid lipofuscin, a complex lipid-protein material, has been reported to accumulate in the cells of HPS patients, predominantly those with HPS1.75
![]() Among the infrequent HPS types, reported symptoms vary. Patients with HPS2 (OMIM #603401) display characteristic clinical findings. The limited number of cases described88,90–154–156 are distinguished by the presence of severe neutropenia together with mild OCA, a mild bleeding diathesis, and a susceptibility to recurrent respiratory infections. Pulmonary function tests are borderline low. The six patients with HPS5 (OMIM #607521) that have been described have not been reported to have significant pulmonary symptoms or colitis.157–159, Patients reported to date with HPS6 (OMIM #607522),158,160 including members of a consanguineous Israeli family with prominent cutaneous hypopigmentation,160 exhibit variable bleeding tendencies without pulmonary or gastrointestinal complications. The single patient reported with HPS7 (OMIM #607145) who was homozygous for a mutation in DTNBP1 complained of some exertional dyspnea, but had objectively normal pulmonary findings in association with a bleeding tendency,161 whereas the members of a Pakistani kindred with HPS8 (OMIM #609762) exhibit only OCA and a bleeding tendency to date.162
Among the infrequent HPS types, reported symptoms vary. Patients with HPS2 (OMIM #603401) display characteristic clinical findings. The limited number of cases described88,90–154–156 are distinguished by the presence of severe neutropenia together with mild OCA, a mild bleeding diathesis, and a susceptibility to recurrent respiratory infections. Pulmonary function tests are borderline low. The six patients with HPS5 (OMIM #607521) that have been described have not been reported to have significant pulmonary symptoms or colitis.157–159, Patients reported to date with HPS6 (OMIM #607522),158,160 including members of a consanguineous Israeli family with prominent cutaneous hypopigmentation,160 exhibit variable bleeding tendencies without pulmonary or gastrointestinal complications. The single patient reported with HPS7 (OMIM #607145) who was homozygous for a mutation in DTNBP1 complained of some exertional dyspnea, but had objectively normal pulmonary findings in association with a bleeding tendency,161 whereas the members of a Pakistani kindred with HPS8 (OMIM #609762) exhibit only OCA and a bleeding tendency to date.162
Mutations in distinct genes, rather than clinical phenotypes, define the various types of HPS. For example, more than two dozen distinct mutations have been found in HPS1 that cause disease.76–78 The most common, found in over 400 Puerto Rican individuals, is a 16-base pair (bp) frameshift duplication in exon 15.79,80 Although the precise function of HPS1 protein is not yet known, HPS1 associates with HPS4 in the 200 kDa BLOC-3 (biogenesis of LROs complex-3) complex81 and has also been found in association with HPS4 in a larger, 500-kDa complex in melanoma cells and fibroblasts.82–84 In melanocytes cultured from the skin of HPS1 patients, the melanogenic enzymes TYR, TYRP1, and DCT/TYRP2 are found in large vesicular structures in the cell body and dendrites, instead of in the granular pattern typically associated with melanosomal localization,85,86 suggesting a role in the control of protein trafficking to the melanosome. Mutations in HPS4 have been described in 15 patients,76 although its exact cellular role is not yet known. Functionally, the ATP-dependent pump MRP4 (ABCC4), normally localized to platelet granules and the plasma membrane, was found to be greatly reduced in HPS4 platelets.87
Mutations or deficiencies in the AP3B1 gene, encoding the β3A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree