
■ Additional imaging studies may be required depending on the functionality of the tumor (metaiodobenzylguanidine [MIBG] for pheochromocytoma in select cases, adrenal vein sampling for hyperaldosteronism, etc.).
■ Fine needle aspiration or core biopsy is not recommended if adrenocortical carcinoma is possible but may be useful if metastasis to the adrenal gland is in the differential and the adrenal tumor is the most accessible site of metastasis in the setting of multiple sites of metastatic disease. If biopsy is performed, it is imperative to first biochemically rule out pheochromocytoma as a diagnosis.
SURGICAL MANAGEMENT
■ Adrenal tumors, if benign appearing by imaging criteria and hormonally nonfunctional, should be reevaluated with at least one additional CT scan or MRI 6 to 12 months after the initial imaging study to ensure stability in size and internal imaging characteristics. Some advocate for a longer period of follow-up imaging for 2 years with reevaluation of biochemistry for 4 more years.1
■ Functional tumors leading to hormone excess, indeterminate masses, and those suspected of being adrenocortical carcinoma or isolated metastatic disease from another primary tumor without evidence of widespread metastatic disease should be removed.
■ Adrenalectomy may be performed by anterior, posterior, or thoracoabdominal approach. The thoracoabdominal approach is particularly useful for providing excellent exposure and access to large adrenal tumors.2–5 Although excellent exposure can be achieved by an anterior approach in most cases, the thoracoabdominal approach is most beneficial in patients who have undergone prior abdominal surgery, thereby avoiding the time and potential morbidity associated with extensive lysis of adhesions.
Preoperative Planning
■ The surgeon must evaluate available imaging studies for possible involvement of adjacent organs and vessels and lymphadenopathy. Invasion of major vessels (vena cava) or adjacent organs may require additional teams or different approaches to the tumor. As CT tends to overestimate invasion of adjacent vessels, MRI with magnetic resonance venogram can be especially helpful to assess for vena cava invasion or intracaval tumor thrombus.
■ The risks, benefits, and alternatives to surgery are discussed with the patient including potential need for steroid supplementation in the postoperative setting. Patients with pheochromocytomas should be adequately alpha blocked and volume replete. Beta blockade may also be necessary. A potassium level should be checked the morning of surgery and treated as necessary in patients with hyperaldosteronism.
■ Routine prophylactic antibiotics and deep vein thrombosis (DVT) prophylaxis are administered prior to surgery, and sequential compression devices are applied.
■ A general endotracheal anesthetic is administered. A dual lumen endotracheal tube may be placed for improved exposure but in general is not required.
■ An epidural is often placed for postoperative pain management.
■ Common to all approaches, skin preparation with clipping rather than shaving is prepared, and the surgical area is prepped and draped in a sterile fashion.
Positioning
■ For a right adrenalectomy, the patient is placed in semi–left lateral decubitus position on a beanbag with an axillary roll beneath the left axilla. The right arm is held in place parallel over the left arm with a thoracic arm holder (FIG 3). For a left adrenalectomy, the opposite setup is performed. The pelvis remains flat. This allows access to the chest and abdomen. The bed is flexed to increase the space between the ribs and the pelvis.

■ All pressure points are padded appropriately.
■ The patient is prepped and draped.
TECHNIQUES
RIGHT ADRENALECTOMY
Exposure
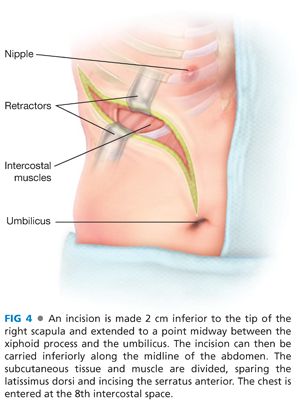
■ An incision is made 2 cm inferior to the tip of the right scapula and extended to a point midway between the xiphoid process and the umbilicus (FIG 4). If necessary, the incision can then be carried inferiorly along the midline of the abdomen. The subcutaneous tissue and muscle are divided, sparing the latissimus dorsi and incising the serratus anterior. The chest is entered at the 8th intercostal space, the pulmonary ligament divided, and the lung retracted superiorly. The diaphragm is incised 2 to 4 cm from its attachments, sparing the phrenic nerve. Several marking stitches should be placed intermittently on either side of the divided diaphragm to aid with reapproximation at the end of the case (FIG 5). The costochondral cartilage is divided. A portion may be excised. The abdominal incision is extended and the peritoneal cavity is entered. A self-retaining retractor system can be placed.

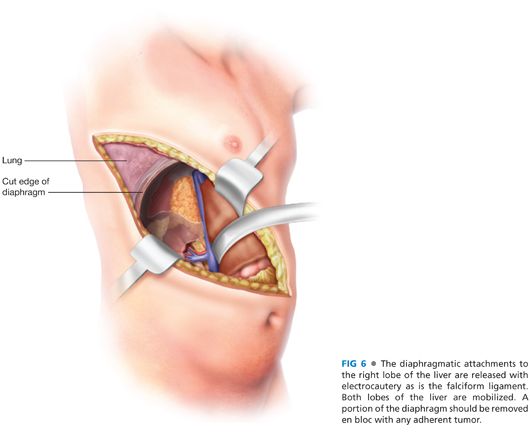
■ The diaphragmatic attachments to the right lobe of the liver are released with electrocautery as is the falciform ligament. The ligamentum teres is ligated and divided with silk ligatures. If the adrenal tumor is adherent to the diaphragm, that portion of the diaphragm should be removed en bloc with the tumor and can be reconstructed with mesh or other material. Inspection of the peritoneal cavity is performed as much as possible in a systematic fashion and an ultrasound of the liver is performed. The right lobe of the liver is retracted toward the left side, allowing access to the retrohepatic inferior vena cava (FIG 6).
Dissection
■ The posterior peritoneal lining over the retroperitoneum in incised over the superior half of the right kidney and carried from medial to lateral out to the side of the abdominal wall (FIG 7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








