Fig. 17.1
Abdominal WAT: the tissue is characterized by large adipose cells and poor collagenic component. (a) Light microscopy (scale bar, 15 μm). (b) SEM (scale bar, 50 μm). (c) SEM (scale bar, 20 μm). The adipocytes are surrounded by a thin capsule of collagen fibers. (d) TEM (scale bar, 2,000 μm). Arrow shows capillary wth stem niche. (e) TEM (scale bar, 500 μm). TEM images confirm that only sparse collagen fibers surround the adipocytes. a adipose cell, c capillary, e endothelium, v vessel
17.7 The Structural WAT (or Type 2 WAT)
With respect to type 1, type 2 adipose tissue is more polymorphous and variable from site to site, as it plays different roles based on the relationships with the surrounding structures. Such variability can also be appreciated as differences in tissue consistency during harvesting. In general, it is a more “stromal” fat and it is located in limited adipose areas, usually rich of muscular tissue.
Type 2 fat is characteristic of more localized depots, where its function goes undoubtedly beyond the storage of lipid itself: in such areas, the adipose tissue appears mainly finalized to mould, which is to give a shape to the structure. The fat in trochanters, sovrapubic area, arm pits, inner faces of the knees, thighs, arms, pectoral and mammary areas and hips all pertain to this group. The average diameter of adipocytes is smaller than in type 1 WAT: about 80–85 μm. Greater diameters can be found in the inner faces of the knees (90–95 μm) and in the trochanters (85–90 μm).
Type 2 fat is a non-lobular adipose tissue with a variable collagenic component, often forming a network around the adipocytes (Fig. 17.2). These cells appear relatively easy to dissociate. The stroma appears generally fairly good and well vascularized and the staminality is also generally fair. Elements related to the basal membrane (poorly differentiated or in course of differentiation) can be found in the pericapillary spaces. Such elements are generally rich of polyribosomes and have few other organules made of isolated endoplasmic reticulum cisternae. In aged people, blood capillaries can show basal membrane duplications or thickenings. Rare macrophagic elements are generally present. Among the various depots, some peculiar aspects can be noticed in those undergoing specific mechanical stimulations in the lower limbs. For example, the depots on the inner faces of the knees and in the trochanteric areas present a quite thin connectival mesh, almost mesenchymal-like. In scan images, the peri-adipocyte baskets are characterized by large meshes made of isolated collagen fibres. Such basket often tends to detach from the plasmalemma of the adipocytes (which in these areas appears uniformly smooth).
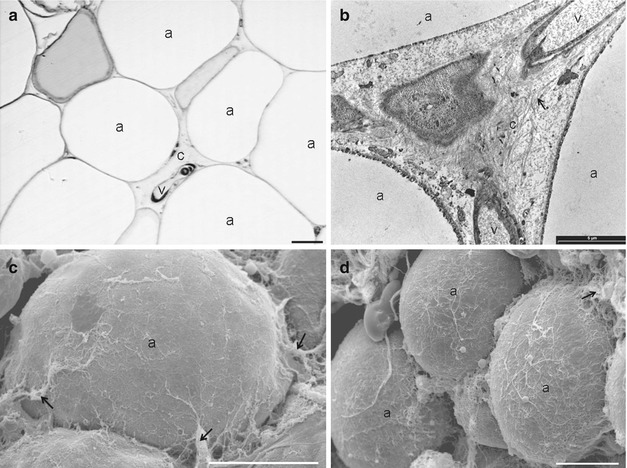

Fig. 17.2
Structural adipose tissue. Type 2 WAT is characterized by a variable collagenic component. (a) Light microscopy (scale bar, 15 μm). (b) TEM (scale bar, 5 μm). The picture shows a stroma rich in collagen fibres and well vascularized. (c) SEM (scale bar, 35 μm). In this area, the connective capsule is thin and partially detached. (d) SEM (scale bar, 20 μm). The picture shows adipocytes covered by a relatively dense connective capsule. a adipose cell, c capillary, v vessel
17.8 The Fibrous WAT (or Type 3 WAT)
Type 3 adipose tissue has a remarkable fibrous component. Probably, this morphologic characteristic is related to a well-defined mechanical function. It can generally be found in areas where the mechanical stress can be considerable. Adipocytes are smaller than in other WAT types and show a thick fibrous shell wrapping them one by one. At SEM, a tight reticulum of thickened collagen fibres is visible in a thick layer around the adipocytes. Such a reticulum can be visible also at optical microscopy, but it appears clearly only at TEM. This kind of adipose tissue, characterized by a conspicuous prevalence of collagenic stroma, can present in two subtypes (i.e. lobular or non-lobular) (Fig. 17.3).
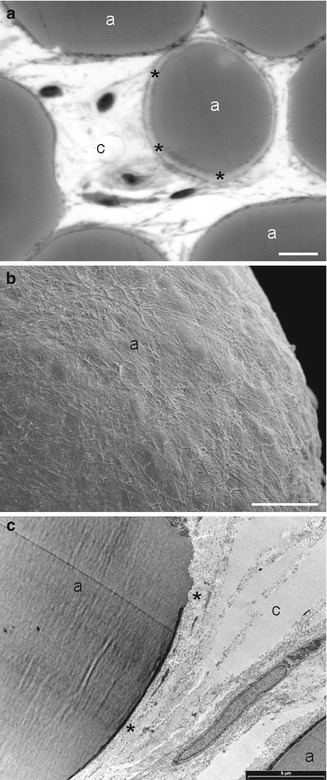

Fig. 17.3
Fibrous adipose tissue with a remarkable fibrous component. (a) Light microscopy (scale bar, 15 μm). (b) SEM (scale bar, 10 μm). A tight reticulum of thickened collagen fibres is visible in a thick layer around the adipocytes. (c) TEM (scale bar, 5 μm). The adipocytes are covered by thick connective sheaths made of very thick collagen bundles. a adipocyte, c connective tissue, v blood vessel, e endothelium, ↑ collagen bundle, * pericellular connective capsule
17.8.1 Lobular Fibrous WAT
This type of WAT can be found in areas in which there is strong mechanical stimulation (e.g. in the calcaneal fat pad). It is a lobular WAT, in which connective septa delimitate micro- and macro-chambers. In the area composed by adipocytes, the tissue is well vascularized, with capillaries characterized by a thick endothelium, in some parts covered by veil cells. The adipocytes are large (90–100 μm in diameter) and covered by thick connective sheaths made of very thick collagen bundles. At SEM, the sheaths appear as compact, high-density layers composed by a tight apposition of collagen fibres, forming a basket-like structure. The outer surface of the basket appears almost regular and easy to dissociate from the stromal collagen. Therefore, the sheaths appear as structures with an evident morphologic identity, closely associated to the plasmalemma of the adipocyte from which, at TEM, they seem to be separated by a virtual space.
17.8.2 Non-Lobular Fibrous WAT
This type of fat is a WAT with a maximum degree of fibrosis but which does not show a lobular organization. It can be found, for example, in the periprosthetic capsules (both irradiated and non-irradiated). It is a hard adipose non-lobulated tissue, with a strong stromal component. The amount of blood vessels is variable but usually not particularly abundant. Around blood vessels, macrophages and elongated elements with a poor lipidic content can be found, which are elements of the adipocyte line in the course of differentiation.
17.9 The Polymorphism of the Subcutaneous WAT
An important data that must be considered during lipofilling procedures is the polymorphism of the subcutaneous WAT. Despite the common idea that fat present an almost homogeneous morphologic pattern, the comparative systematic analysis of human samples demonstrated that the WAT appears as a very differentiated structure. This leads to the realization that it should be more appropriate to use the term subcutaneous WATs instead of subcutaneous WAT. The data obtained by groups of patients of different ages suggest that such a specialization could be in part related to the length of human life. In the authors’ experience, the difference among the various lipid depot is less evident in the common laboratory species (i.e. rodents), whose average lifespan is generally no longer than 24 months. It appeared to be very difficult to appreciate these differences at optical microscopy and this is probably the main reason why they have never been underlined in studies led with different aims. The difference among the various types of WAT seems to be mainly related to the composition of the stromal compartment and it patently emerged that these differences could be substantially connected to the functional specializations of the depots in the various sites. These results about regional differences in morphology confirm the data indicating that there are regional differences in the metabolism of subcutaneous fatty depots [21, 22]. Lipids are mobilized at a slower rate but synthesized at a higher rate in the femoral than the abdominal region. Fasting is accompanied by an increased rate of fat mobilization and a decreased rate of fat synthesis in all fatty depots. These changes are, however, more pronounced in abdominal than in femoral fat. There are also regional differences in the hormonal regulation of fat metabolism in obesity. The action of insulin is most pronounced in the femoral region, whereas the one of catecholamines is most marked in the abdominal area. The regional differences in hormone action are further enhanced during therapeutic fasting [21]. Abdominal adipocytes from females showed a 40 times lower alpha 2-adrenergic antilipolytic sensitivity than did gluteal adipocytes [22].
17.10 The Importance of the Stroma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree